Practice Test 3 - AP Chemistry Premium 2024
Section I: Multiple-Choice
Start only when you have 90 minutes to complete the whole section.
.png)
Instructions
There are 60 multiple-choice questions for this part of the exam. Enter your answers on the answer sheet provided. On the actual exam, no credit will be given for answers marked on the test itself. For this test, mark your selected answer next to the question and then transfer it to the scoring sheet. (This will allow you to check that all answers are properly transferred to the scoring sheet.) Each question has only one answer. When changing answers, be sure to erase completely.
Useful Hints
Not everyone will know the answers to all of the questions. However, it is to your advantage to provide an answer to all questions. Do not waste time on difficult questions. Answer the easier ones first, and return to the difficult ones you have not answered if time remains.
Your total score is simply the number of questions answered correctly. Wrong answers or blanks on the scoring sheet do not count against you.
You will be allowed to use the following periodic table and the table of equations and constants on this part of the test.
.png)
.png)
.png)
.png)
.png)
Section I
60 Multiple-Choice Questions
(Time: 90 minutes)
CALCULATORS ARE ALLOWED FOR SECTION I
Note: For all questions, assume that T = 298 K, P = 1.00 atmosphere, and H2O is the solvent for all solutions unless the problem indicates a different solvent.
1. An experiment was performed to determine the moles of carbon dioxide gas formed (collected over water) when an acid reacts with limestone. To do this, a piece of dry limestone was weighed. Then carbon dioxide was collected until the limestone disappeared. The atmospheric pressure was measured at 0.965 atm. The temperature was 295 K. The volume of CO2 was measured to the nearest mL and corrected for the vapor pressure of water. The student continuously got low results from what was expected. Can you suggest why?
(A) Limestone is never pure CaCO3.
(B) Limestone is Ca(HCO3)2.
(C) Carbon dioxide is rather soluble in water.
(D) Perhaps there was not enough acid to dissolve all the limestone.
.png)
2. Choose the description of the point on the distribution curve that is the most plausible.
(A) Point A represents absolute zero where all motions stops.
(B) Point C is proportional to temperature
(C) Point B is where the transition state occurs.
(D) At point D, the products of the reaction are found.
3. Your supervisor asks you to determine the enthalpy of a certain chemical reaction. Which would you do?
(A) Measure the ∆S and the ∆G for the reaction, and calculate the ∆H from the Gibbs free-energy equation.
(B) Use a bomb calorimeter to measure the heat of the reaction.
(C) Use a solution calorimeter such as a coffee-cup calorimeter to measure the heat.
(D) Use a photoelectron spectrometer to measure the energies of all atoms in the compounds, and use Hess’s law to add them.
4. A 25 g sample of a solid was heated to 100 °C and then quickly transferred to an insulated container holding 100 g of water at 26 °C. The temperature of the mixture rose to reach a final temperature of 37 °C. Which of the following can be concluded?
(A) The sample lost more thermal energy than the water gained because the sample temperature changed more than the water temperature did.
(B) Even though the sample temperature changed more than the water temperature did, the sample lost the same amount of thermal energy as the water gained.
(C) The sample temperature changed more than the water temperature did; therefore, the heat capacity of the sample must be greater than the heat capacity of the water.
(D) The final temperature is less than the average starting temperatures; therefore, the equilibrium constant must be less than 1.
.png)
5. Three 1-liter flasks are connected to a 3-liter flask by valves as shown in the diagram above. The 3-liter flask has the relative number of helium atoms as indicated. At the start, the entire system is at 585 K. The first flask contains oxygen, the second contains hydrogen, and the third contains nitrogen. The pressure of hydrogen is 3.00 atm. The number of gas molecules is proportional to their representations in the flasks. If the valves are all opened, what will be the pressure in the system? Assume the connections have negligible volume.
(A) 1.0 atm
(B) 2.0 atm
(C) 3.0 atm
(D) 4.0 atm
.png)
6. A mass spectrum of a naturally occurring sample of an element is shown above. What is the element?
(A) Cl
(B) S
(C) Ar
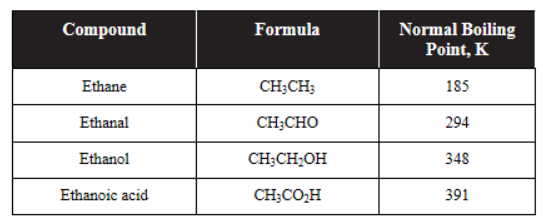
(D) There are two peaks, so there must be two compounds.
7. Given the following weak bases with their respective Kb, which sequence lists the corresponding cations in order of increasing acid strength?
.png)
8. A laboratory assistant was told to create a buffer solution with a pH equal to 5. Which of the following acids would be the best choice?
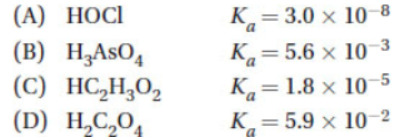
9. A student is performing a titration of a diprotic weak acid, H2A (0.10 mol L-1), with a strong base of the same concentration. Which of the following is the best representation of the resulting titration curve?
.png)
.png)
.png)
10. A student uses a pH indicator that changes color from pH 8 to 6. Which statement best characterizes the expected observations based on the titration curve above?
(A) The color will change slowly, and the end point volume will be low.
(B) The observed color change will be distinct, and the calculated molarity of the base will be accurate.
(C) The color will change slowly, and the calculated molarity of the base will be low.
(D) The observed color change will be distinct, but the calculated molarity of the base will be high.
11. When performing a titration of 0.050 M benzoic acid, C6H5COOH, (Ka = 6.6 × 10-5) with 0.050 M NaOH, which of the following indicators would be the best choice?
(A) Bromphenol blue, pH range 3.0-4.5
(B) Phenolphthalein, pH range 8.0-10.1
(C) Phenol red, pH range 6.9-8.2
(D) Bromcresol green, pH range 3.8-5.4
Questions 12-14 refer to the information and graph below.
Four different 0.0100 M acid solutions were prepared, and their pH values were recorded on a laptop computer. One of the solutions contains more than just an acid. At the point designated as “add,” all four solutions are diluted with an equal volume of water. The bottom line represents solution 1, and the top line represents solution 4.
.png)
12. Using the data in the graph, which of the solutions does not contain just an acid dissolved in water?
(A) Solution 4 because the pH does not change upon dilution
(B) Solution 1 because the pH changes too much
(C) Solutions 2 and 3 because the pH did not change enough
(D) Solution 4, which must be pure water since the pH does not change
13. Using the data presented in the graph and the experiment that was performed, which of the weak acids is the weakest?
(A)Solution 1 because its pH changed the most
(B)Solution 4 because the pH did not change at all
(C)Solution 2 because its pKa appears to be about 3.20
(D)Solution 3 because it had the highest pH of all the solutions made with 0.0100 M acid
14. Which of the acids will react with copper metal?
(A) Acid 1 and acid 3
(B) We cannot tell; we need to know what the cations are.
(C) Acid 2
(D) We cannot tell; we need to know what the anions are.
15. Chemists often ascribe the macroscopic properties of solids to the underlying microscopic structure. Silicon carbide is almost as hard and brittle as diamond. The solid state structure of silicon carbide is often described as
(A) a molecular crystal
(B) a covalent or network crystal
(C) a metallic crystal
(D) an ionic crystal
.png)
16. In the diagram above, which labeled arrow is pointing toward a covalent bond and which is pointing toward a hydrogen bond?
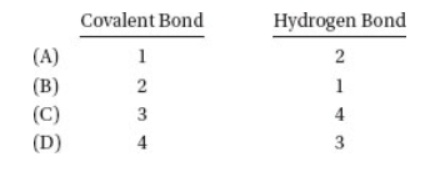
17. Which is the easiest way to burn a silver coin?
(A) Hold the silver coin with crucible tongs, and heat strongly in the flame of a Bunsen burner.
(B) Use the method in (A), but use an oxyacetylene torch to reach a higher temperature.
(C) Grind the silver coin into very small, dust-sized particles, and spray the particles into a Bunsen burner flame.
(D) Dissolve the silver coin in acid, precipitate the hydroxide, and heat in a Bunsen burner flame to make the oxide.
Questions 18-21 refer to the table below.
.png)
Note that in addition to the data in the table, the gases are held in separate, identical, rigid vessels.
18. Which sample has the lowest density?
(A) All have the same density since the temperatures are all the same.
(B) Vessel A
(C) Vessel B
(D) Vessel C
19. The average kinetic energy
(A) is lowest in vessel A
(B) is lowest in vessel B
(C) is lowest in vessel C
(D) is the same in all vessels
20. Which of these gases is expected to condense at the lowest pressure, assuming that the temperature is held constant?
(A) Xenon
(B) Argon
(C) Neon
(D) They all condense at the same pressure.
21. Which attractive force is the major cause of condensation of the three compounds in the table above?
(A) Hydrogen bonding
(B) London forces
(C) Dipole-dipole attractive forces
(D) Molar mass
.png)
22. Use the arrangement of atoms suggested in the skeleton structure above to construct the Lewis structure for the SO3 molecule. All of the following statements about this molecule are true EXCEPT
(A) sulfur trioxide has three resonance structures
(B) sulfur trioxide has a planar triangle shape
(C) sulfur trioxide is nonpolar
(D) the S-O bond order is 5/3
.png)
23. Use the arrangement of atoms suggested in the skeleton structure above to construct the Lewis structure for the sulfite ion. Be sure to minimize the formal charges. Which statement is the least likely to be true?
(A) The sulfite ion has a double bond.
(B) The sulfite ion has a triangular pyramid shape.
(C) The sulfite ion is nonpolar.
(D) The S-O bond order is 4/3.
S(s) + O2(g) → SO2(g)
24. The reaction between sulfur and oxygen, written in equation form above, can be interpreted in all of the following ways EXCEPT
(A) one atom of S reacts with one molecule of O2 to yield one molecule of SO2
(B) one mole of sulfur atoms reacts with one mole of oxygen molecules to yield one mole of sulfur dioxide molecules
(C) the position of equilibrium must be on the product side
(D) the entropy increase will be large
Questions 25-26 refer to the following diagram.
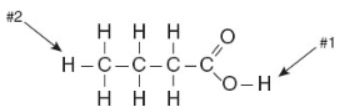
25. Which hydrogen in butanoic acid, shown above, will ionize and why?
(A) Hydrogen #1: it is at the end of the formula.
(B) Hydrogen #1: its bond to the rest of the molecule is the weakest due to the electronegativity of two nearby oxygen atoms.
(C) Hydrogen #2: there is a 7-to-1 probability of the hydrogens bound to carbon ionizing.
(D) Hydrogen #2: this type of H is very electronegative.
26. Butanoic acid C4H8O2, shown above, gives rancid butter its foul smell. It has a Ka = 1.5 × 10-5. If a 0.0100 M solution of butanoic acid is prepared, the expected pH will be in which one of the following pH ranges?
(A) 2 to 4
(B) 4 to 6
(C) 8 to 10
(D) 10 to 12
Questions 27-29 refer to the following equation.
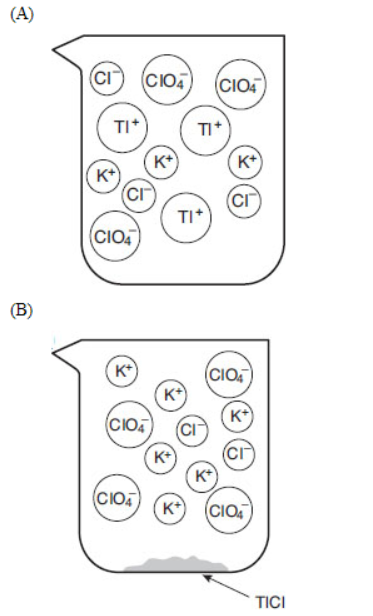
Tl+(aq) + Cl-(aq) → TlCl(s)
A chemist mixes a dilute solution of thallium perchlorate with a dilute solution of potassium chloride to precipitate thallium chloride (Ksp = 1.9 × 10-4).
27. Which of the following is a molecular equation for the reaction described above?

28. If equal volumes of 2.0 millimolar solutions are mixed, which of the following particulate views represents the experiment after the reactants are mixed thoroughly and solids are given time to precipitate?
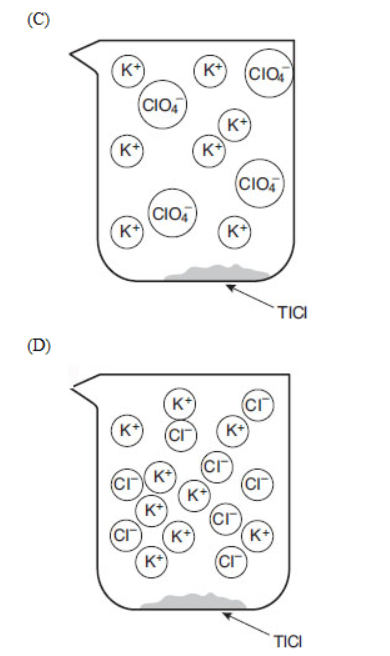
29. Within a factor of 10, what is the approximate molar solubility of thallium chloride in distilled water?
(A) 10-2 mol/L
(B) 1.8 × 10-5 mol/L
(C) 10-7 mol/L
(D) 10-15 mol/L
Questions 30-35 refer to the information and graph below.
O2(g) + 2SO2(g) ⇌ 2SO3(g)
SO2(g) is produced in the combustion of coal, oil, gasoline, and many other natural products. In the atmosphere, it reacts with oxygen to form SO3(g) via the equation above.
A pure sample of SO3(g) is placed into a rigid, evacuated 0.500 L container. The initial pressure of the SO3(g) is 760 torr. The temperature is held constant until the system reaches equilibrium. The figure below shows how the pressure of the system changes in reaching equilibrium.
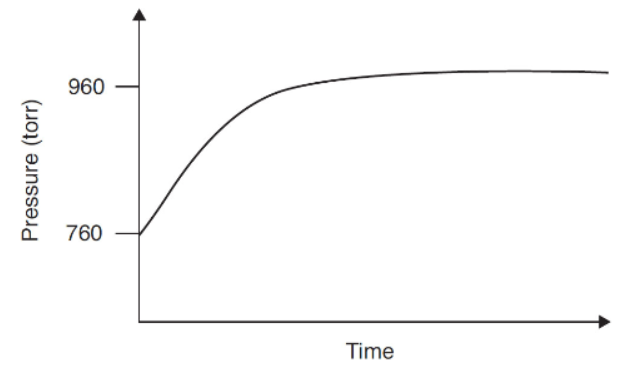
30. Why does the pressure rise in this experiment?
(A) Actually, the pressure should decrease since three moles of gas are producing two moles in the reaction.
(B) SO2 is polar, resulting in more effective collisions with the container walls.
(C) The nonpolar sulfur trioxide becomes the polar sulfur dioxide, thus increasing the number of collisions per second.
(D) Two moles of SO3 are producing three moles of gas, increasing the particles in the container colliding with the walls.
31. The pressure versus time curve can be used to
(A) determine the rate law for the reverse reaction
(B) determine the rate law for the forward reaction
(C) determine the activation energy for the reverse reaction
(D) determine the enthalpy of this gas phase reaction
32. What will the final pressure be if this reaction goes to completion?
(A) 760 torr
(B) 900 torr
(C) 1140 torr
(D) 2280 torr
33. Sulfur trioxide decomposed to form some sulfur dioxide and molecular oxygen. What is the proper form for the equilibrium expression for the reaction that actually occurred?
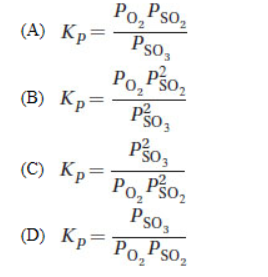
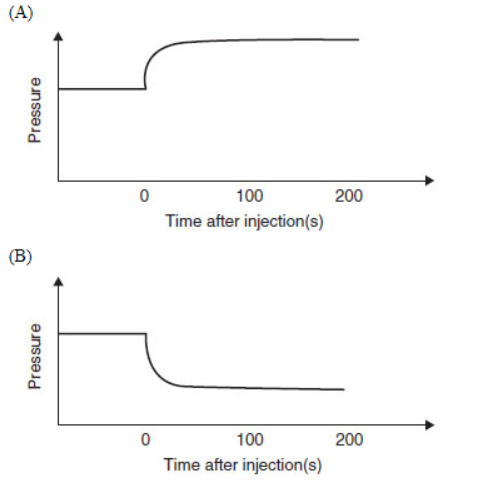
34. Which diagram is an appropriate description of the system if more SO2(g) is rapidly injected, at time = 0, into the container after equilibrium is established?
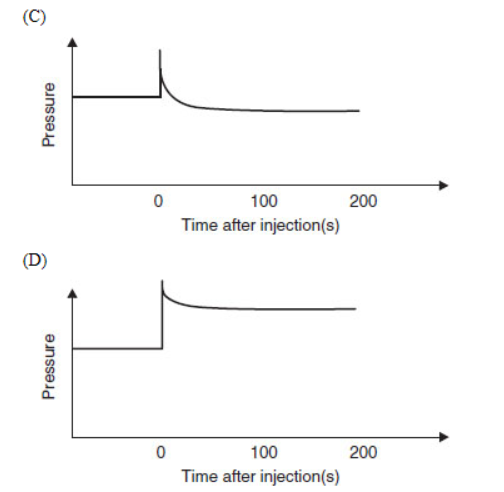
35. Why are SO2(g) and SO3(g) pollutants to be concerned about?
(A)They contain yellow sulfur that causes jaundice.
(B)They both are acid anhydrides, forming H2SO3 and H2SO4, which are a major part of acid rain.
(C)They both have resonance structures that lead to free radicals that cause cancer.
(D)They create the brown layer of smog seen over many cities and destroy O3, which is essential for life.
________________________________________________________________________________________
36. Fluorine has a normal boiling point of 85 K, and chlorine boils at 239 K. The Cl2 molecule is much larger than F2 (atomic radius is 99 pm for chlorine and is 64 pm for fluorine). Which is the best reason for the large difference in boiling points?
(A) Chlorine has a higher dipole moment than fluorine.
(B) The intramolecular bonds in Cl2 are much weaker than those in F2.
(C) The Cl2 electron clouds are much more polarizable than the F2 electron clouds, resulting in much stronger London forces.
(D) The mass of chlorine is much greater than the mass of fluorine.
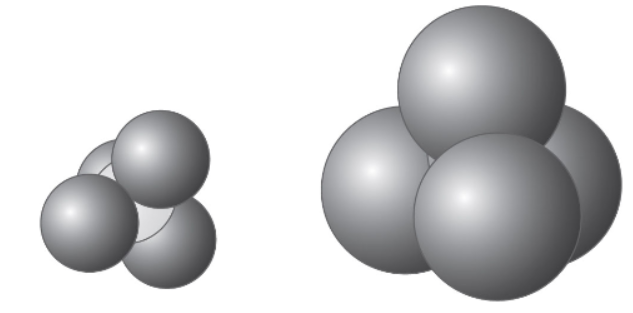
37. Space-filling representations of carbon tetrachloride and carbon tetrabromide are shown above. The carbon can be seen in the carbon tetrachloride and is hidden by the bromine in carbon tetrabromide. Which of the following is the most reasonable statement? (Assume that the temperatures of CCl4 and CBr4 are the same in these comparisons.)
(A) CCl4 has a higher surface tension compared with CBr4.
(B) CCl4 has a higher vapor pressure compared with CBr4.
(C) CCl4 has a higher boiling point compared with CBr4.
(D) CCl4 has a higher viscosity compared with CBr4.
Refer to the following thermochemical reactions and heats of reaction.

38. What is the enthalpy of the reaction 2NO2 → N2 + 2O2?
(A) -294.0 kJ
(B) -67.6 kJ
(C) +294.0 kJ
(D) The enthalpy of reaction cannot be determined with this information.
39. Select the balanced chemical equation for the combustion of ethyl alcohol, C2H5OH(l).
(A) C2H5OH(l) + 5O2(g) → 2CO2(g) + 3H2O(l)
(B) 4C2H5(l) + 13O2(g) → 8CO2(g) + 10H2O(g)
(C) 2C2H5O(l) + 9O2(g) → 4CO2(g) + 5H2O(g)
(D) C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(g)
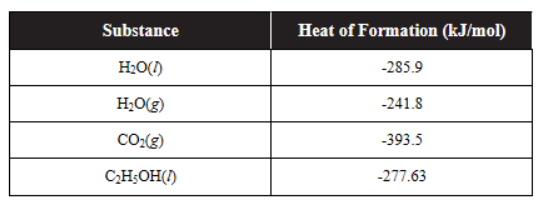
40. Using the table of heats of formation above, determine the heat of combustion for ethyl alcohol, C2H5OH(l).
(A) 2(-277.63) + 7(0.00) - 4(-393.5) - 6(-241.8)
(B) 2(-393.5) + 3(-241.8) - (-277.63) - 3(0.00)
(C) 4(-393.5) + 5(-241.8) - 2(-277.63) - 9(0.00)
(D) 4(-277.63) + 13(0.00) - 8(-393.5) - 10(-241.8)
41. Using the table of heats of formation above, calculate the heat of vaporization of water.
(A) (-241.8) - (-285.9)
(B) (-285.9) - (-241.8)
(C) (241.8) - (-285.9)
(D) (-285.9) - (241.8)
42. Can the standard entropy change for the vaporization of water at 298 K be determined?
(A) No, because the equilibrium constant and Q are not known.
(B) Yes, it is equal to 1/∆H°vap.
(C) Yes, a phase change from liquid to gas or solid to liquid always has ∆S° = ٠.٠٠.
(D) Yes, since ∆G° is zero for water and steam at this temperature, then ∆S° must be equal to ∆H°vap/T.
43. A chemical system at standard state has all concentrations at 1 M and therefore Q = 1.00. If the standard cell potential is +0.500 V, which of the following is false?
(A) The reaction is thermodynamically favored, and the reaction proceeds in the forward direction.
(B) The reaction is thermodynamically favored, and the concentrations of the products increase.
(C) The reaction is thermodynamically favored, and the concentrations of the reactants decrease.
(D) The reaction is thermodynamically favored, and the reaction proceeds in the reverse direction, increasing the concentration of reactants.
Questions 44-46 refer to the galvanic cell shown below. The voltmeter reads 0.658 V.
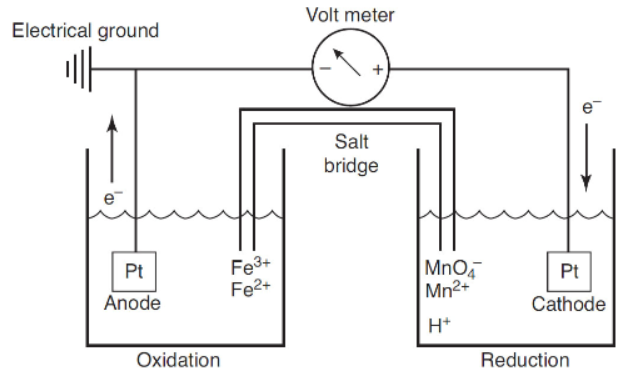
44. What is the cell diagram for the cell above?
(A) Pt | Fe2+, Fe3+ || MnO4-, H+, Mn2+ | Mn
(B) Pt | Fe2+ | Fe3+ || MnO4-, H+ | Mn2+ | Pt
(C) Pt | Fe2+, Fe3+ || MnO4-, H+, Mn2+ | Pt
(D) Fe2+ | Fe3+ || MnO4-, H+
45. Which balanced chemical equation is thermodynamically favored?
(A) Fe2+(aq) + MnO4-(aq) + H+(aq) → Mn2+(aq) + Fe3+(aq) + H2O
(B) 5Fe3+(aq) + MnO4-(aq) + 8H+(aq) → Mn2+(aq) + 5Fe2+(aq)
(C) 5Fe2+ + MnO4- + 8H+ → Mn2+ + 5Fe3+ + 4H2O
(D) 5Fe3+ + MnO4- + 8H+ → Mn2+ + 5Fe2+
46. For the galvanic cell shown in the diagram, what will be observed if the voltmeter is replaced by a copper wire?
(A) The purple color of the permanganate ion will slowly disappear.
(B) The purple color of the permanganate will increase.
(C) Nothing happens without the voltmeter being present.
(D) The pH of the left cell will not change, but the pH will decrease in the right cell.
The first ionization energy of sodium is 496 kJ/mol, and its atomic radius is 186 pm.
47. Based on the information above and your knowledge of periodic trends, which values are the most reasonable for the radius and first ionization energy of the magnesium atom?
(A) 160 pm, 737 kJ/mol
(B) 86 pm, 398 kJ/mol
(C) 235 pm, 523 kJ/mol
(D) 240 pm, 1200 kJ/mol
48. Dissolving one mole of each of the oxoacids HNO2, HClO4, H2CO3, and H3PO4 in 2.0 L of distilled water results in solutions with different pH values. Arrange these acid solutions from the one with the highest pH to the one with the lowest pH.
(A) HNO2 > HClO4 > H2CO3 > H3PO4
(B) HClO4 > HNO2 > H2CO3 > H3PO4
(C) H2CO3 > H3PO4 > HNO2 > HClO4
(D) H2CO3 > HNO2 > HClO4 > H3PO4
49. Based on the data in the table above, which of the following substances has lowest vapor pressure at any given temperature?
(A) Ethane
(B) Ethanal
(C) Ethanol
(D) Ethanoic acid
50. Based on periodic relationships, the bond strength, and the concept relating bond strength to acid strengths, which of the following correctly predicts the strength of binary acids from strongest to weakest?
(A) H2Se > H2O > H2S
(B) H2Se > H2S > H2O
(C) H2O < H2Se < H2S
(D) H2O > H2S > H2Se
51. Silver metal, often amalgamated with mercury, is used to reduce substances to a desired oxidation state. If the silver metal amalgam cannot be used because of concerns about mercury, which of the following would be a reasonable and safe substitute?
(A) H+(aq)
(B) Na(s)
(C) Ca2+(aq)
(D) Mg(s)
52. Which of the following particulate diagrams best represents the reaction of carbon with oxygen to form carbon dioxide?
53. 0.25 mol of a weak, monoprotic acid is dissolved in 0.25 L of distilled water. The pH was then measured as 4.26. What is the pKa of this weak acid?
(A) 8.52
(B) 7.52
(C) 4.26
(D) 3.66
54. Sulfurous acid is a weak acid, while sulfuric acid is a much stronger acid because
(A) the O-H bonds in sulfuric acid are much weaker than in sulfurous acid due to the electron withdrawing of the oxygen atoms on sulfuric acid
(B) sulfuric acid has more oxygen atoms in its formula
(C) the sulfur in sulfuric acid is more electronegative than the sulfur in sulfurous acid
(D) sulfuric acid has the hydrogen atoms bound directly to the sulfur atom
55. To prepare a buffer, all of the following are needed EXCEPT
(A) an acid with a pKa close to the desired pH
(B) a conjugate acid along with its conjugate base
(C) a buffer capacity sufficient to react with added acid or base
(D) triple-distilled water
The photoelectron data for sodium are shown below.
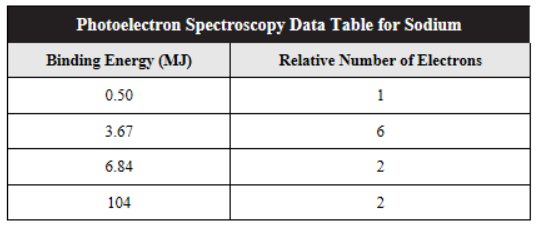
56. Which of the following statements is not true about these data?
(A) All 11 electrons are shown in the table.
(B) The 3 valence electrons have the lowest energies at 0.50, 3.67, and 6.84 MJ/mol.
(C) The p electrons are all at the same energy of 3.67 MJ/mol.
(D) Electrons closest to the nucleus have the highest energies.
57. Identify the Brønsted-Lowry conjugate acid-base pair in the following list.
(A) H3O+ and OH-
(B) H3PO4 and H2PO4-
(C) HClO4 and ClO3-
(D) SO32- and HSO2-
58. Chemical reactions can be classified as either heterogeneous or homogeneous. Which of the following equations below is best classified as a heterogeneous reaction?
(A) 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(g)
(B) C2H5OH(aq) + O2(aq) → HC2H3O2(aq) + H2O
(C) C(s) + H2O(g) → H2(g) + CO(g)
(D) C2H2(g) + 5N2O(g) → CO2(g) + H2O(g) + 5N2(g)
59. Which of the following may need to be balanced using the ion-electron method?
(A) BaCl2 + Al2(SO4)3 → AlCl3 + BaSO4
(B) H+ + OH- → H2O
(C) NaOH + H3PO4 → Na2HPO4 + H2O
(D)C2H2(g) + N2O(g) → CO2(g) + H2O(g) + N2(g)
60. Which of the reactions below will become thermodynamically favored only at high temperatures?
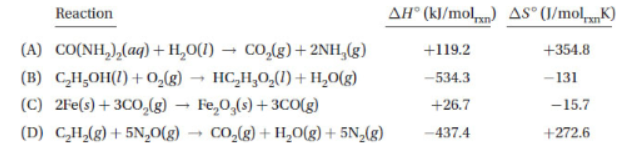
.png)
Section II: Free-Response
Start only when you have 105 minutes to complete the whole section.

Instructions
The questions appear on the following pages. You may use the periodic table and the table of equations and constants that were provided with Section I. On the actual exam, you will be directed to write your final answers only in the test booklet. For this practice test, write your answers on separate sheets of lined paper. You will need about three pages for each of questions 1 through 3 and one page for each of questions 4 through 7.
Do not spend too much time on any one question. Budget your time carefully, and answer the easier questions first.
Be sure that your work is well organized, complete, and easy to read. Cross out or erase any mistakes. Erased and crossed-out material will not be scored.
Section II
7 Free-Response Questions
(Time: 105 minutes)
CALCULATORS ARE ALLOWED FOR SECTION II
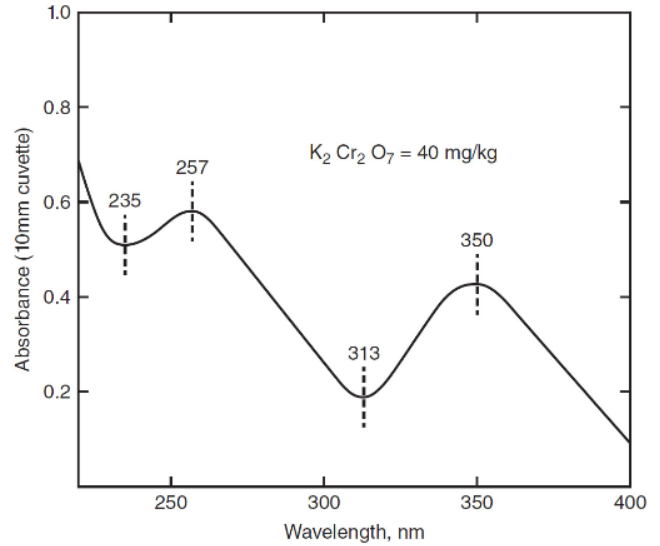
1. Students in a class are tasked with creating 500. mL of a standard solution of 1.000 M potassium dichromate from a 5.000 M solution of K2Cr2O7 and then use that solution to make five 50.0 mL solutions for the absorbance calibration plot with 5 data points that range from 0.02 M to 0.10 M.
(a) Circle the items absolutely needed for this task and draw a line through necessary safety equipment:

(b) What are the labels for the two axes of a calibration plot?
(c) When constructing the calibration plot, what kind of data is considered to be independent so that it would be on the independent axis?
(d) Which axis (x or y) is traditionally used for the dependent data?
(e) Fill in the blanks with correct words, but do not use the words “independent” or “dependent.”
For a calibration plot, an appropriate label for the x-axis is _________________________. For that same calibration plot, an appropriate label for the y-axis is ______________________________________.
(f) What is the minimum number of independent dilutions from the original 5.000 M solution that are needed for a 5-point calibration plot?
(g) The spectrum for potassium dichromate in the ultraviolet spectral region is shown at the beginning of the problem. What wavelength should be used for the analysis of potassium dichromate? Explain your why you chose this wavelength. Refer to the spectrum for dichromate.
(h) For one analysis, the peak at 257 nm was used to prepare the calibration curve. A second analysis used the 350 nm peak for the calibration curve. Which calibration curve would have the larger slope? Explain your reasoning.
2. The titration with an acidic solution is undertaken to precisely determine the concentration of a solution of a base. Below is an illustration of the buret used before the titration was started and after it was completed. The phenolphthalein indicator was observed changing from pink to colorless. The sample was pipetted into the reaction vessel using a 50 mL volumetric pipet.
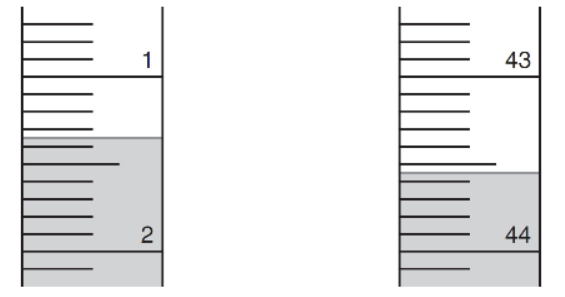
(a) Base your answers to the following on the reading of the acid in the buret before the titration was started.
(i) What was the initial volume? _____________mL
(ii) How many digits are certain? __________
How many digits are estimated? _________
(iii) How uncertain is this reading? +/- mL
(b) Base your answers to the following on the end point volume.
(i) What is the end point volume from the figure? _____________mL
(ii) How many significant figures does this result have? _______
(c) Calculate the total volume of titrant added to the reaction vessel.
(d) Calculate the concentration of the alkaline water.
(i) Show your calculation of the concentration of base in the sample.
(ii) What is the pH of the sample?
(e) What is the purpose of a primary standard?
(f) What is the purpose of standardization in a titration experiment?
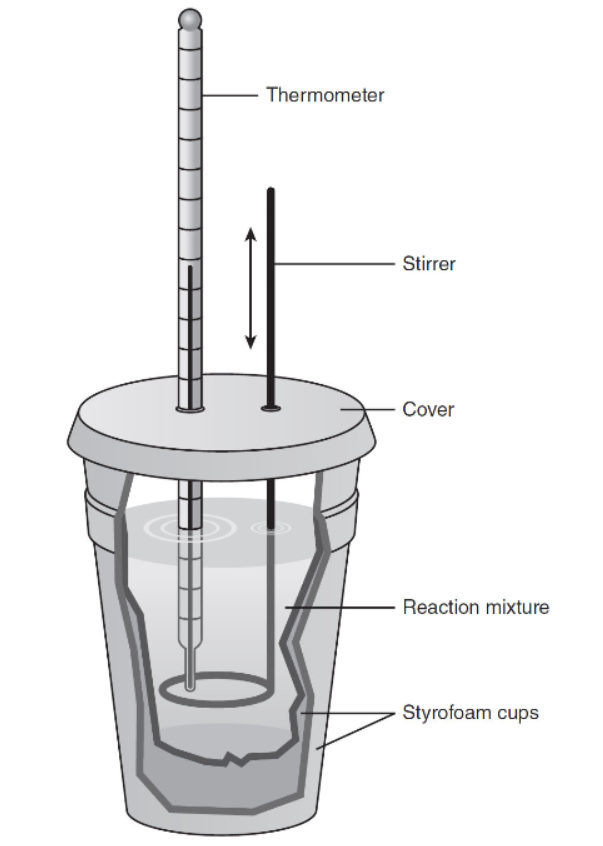
3. Calorimetry is a method to measure the enthalpy change, ΔHreaction, of a reaction. You may have performed an experiment using a “coffee-cup” calorimeter. It consists of two foam polystyrene cups nested together, with a cap made of foam polystyrene that was cut to fit the top to help keep heat inside as shown in the diagram.
Modern calorimeters often have an electronic temperature sensor connected to a computer that records the temperature change on a graph or spreadsheet. One reactant is placed into the calorimeter, and the second is added quickly as temperature readings are collected. The basic calorimetry equation is
q = (specific heat)(mass)(Tfinal - Tinitial)
(a) What is specific heat? What are its units? Why is specific heat needed?
(b) The specific heat of water is 4.184 J g-1 K-1, but that same value is also used for dilute solutions. Why is that reasonable?
(c) How can the enthalpy of the reaction of hydroxide ions (OH-) and hydronium ions (H3O+) be implemented?
(d) How can the enthalpy of ionization of propanoic acid be determined?
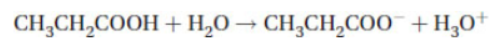
(e) The formation equation for propanoic acid is
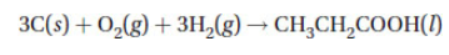
The combustion of propanoic acid is shown with the following equation:
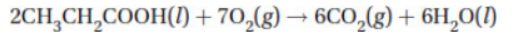
The combustion of carbon and hydrogen reactions are
.png)
Suggest how the heat of formation of propanoic acid can be determined. Do not do the calculations or the combining of equations; just suggest the process.
4. In many chemical processes, oxidation and reduction occur together in a chemical reaction. The term redox was coined to emphasize this pairing. The following is the skeleton for a redox reaction. The questions that follow all refer to it.
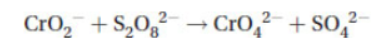
(a) Oxygen in the skeleton reaction always has a -2 oxidation number. Place the oxidation numbers for all Cr and S atoms above their symbols in the equation.
(b) Balance the equation in a basic solution. Show your method.
(c) Which substance is oxidized in the reaction? Justify your selection.
(d) Sodium (Na+) ions and chloride (Cl-) ions were the spectator ions. Write the molecular reaction.
5. Phosphoric acid, a flavoring agent in many colas and other soft drinks, has three ionizable protons.
(a) Write and balance the three ionization reactions for H3PO4. Include the Ka for each ionization step.
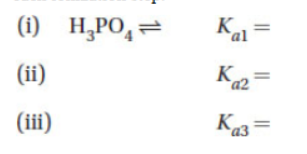
(b) Add the three reactions in part (a) to obtain the total ionization of phosphoric acid.
(c) Write the overall equilibrium constant Koverall for your result.
(d) Show by cancellation that Koverall = Ka1 × Ka2 × Ka3.
6. Below is a simplified photoelectron spectrum of a pure element.
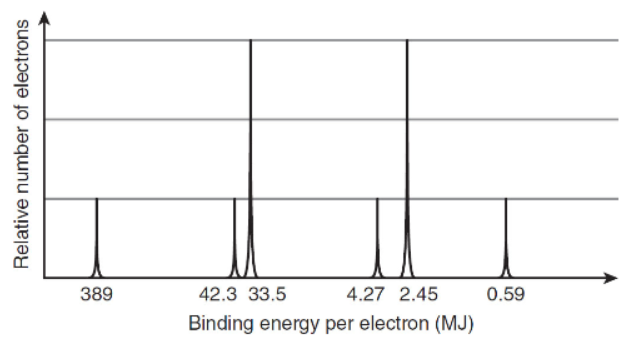
(a) What is the first ionization energy of this substance?
(b) Write the ground state electron configuration for this element.
(c) Identify the element in this experiment.
(d) The 9th and 10th ionization energies for the two 3s electrons are different. However, in the photoelectron spectrum, they have the same binding energy. Explain why.
7. Below is a plot of the number of molecules of octylacetate, CH3COO(CH2)CH3, an ester that has the odor of oranges, versus the kinetic energy of the molecules in a liquid at 35 °C.
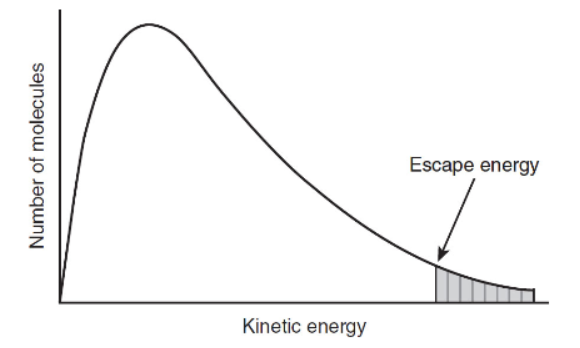
(a) Describe the concept of energy as related to the escape energy.
(b) Sketch a curve on the figure above for this sample after it has been increased to a temperature of 50 °C.
(c) Can your curve in part (b) explain why the odor of oranges is stronger at an elevated temperature?
(d) What point on each curve represents a direct relationship to the temperature?


.png)



