Practice Test 2 - AP Chemistry Premium 2024
Section I: Multiple-Choice
Start only when you have 90 minutes to complete the whole section.

Instructions
There are 60 multiple-choice questions for this part of the exam. Enter your answers on the answer sheet provided. On the actual exam, no credit will be given for answers marked on the test itself. For this test, mark your selected answer next to the question and then transfer it to the scoring sheet. (This will allow you to check that all answers are properly transferred to the scoring sheet.) Each question has only one answer. When changing answers, be sure to erase completely.
Useful Hints
Not everyone will know the answers to all of the questions. However, it is to your advantage to provide an answer to all questions. Do not waste time on difficult questions. Answer the easier ones first, and return to the difficult ones you have not answered if time remains.
Your total score is simply the number of questions answered correctly. Wrong answers or blanks on the scoring sheet do not count against you.
You will be allowed to use the following periodic table and the table of equations and constants on this part of the test.
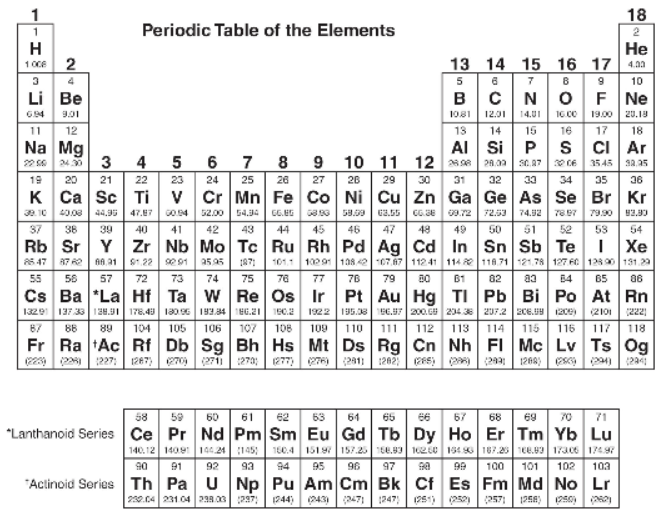
Section I
60 Multiple-Choice Questions
(Time: 90 minutes)
CALCULATORS ARE ALLOWED FOR SECTION I
Note: For all questions, assume that T = 298 K, P = 1.00 atmosphere, and H2O is the solvent for all solutions unless the problem indicates a different solvent.

Questions 1–4 refer to the following information.
Four different acid solutions are prepared, and their pH values are determined and tabulated below.
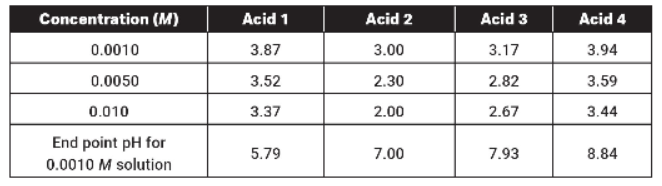
1. Using the data in the table above, which is the weakest acid?
(A) Acid 1
(B) Acid 2
(C) Acid 3
(D) Acid 4
2. Which of the four acids is best described as a strong acid?
(A) Acid 1
(B) Acid 2
(C) Acid 3
(D) Acid 4
3. Which of the following operations will produce an effective buffer solution?
(A) 50.0 mL of 0.010 M acid 3 mixed with 50.0 mL of 0.010 M acid 2
(B) 50.0 mL of 0.010 M acid 2 mixed with 25.0 mL of 0.010 M NaOH
(C) 50.0 mL of 0.0050 M acid 4 mixed with 0.25 mL of 0.0050 M NaOH
(D) 50.0 mL of 0.0010 M acid 2 mixed with 55.0 mL of 0.0010 M NaOH
4. Each of the acids were titrated with NaOH to the end point. The end-point pH values are shown on the last line of the table. The end-point pH values
(A) suggest that acid 3 is strong and all the rest are weak
(B) suggest that all the acids are weak
(C) suggest that acid 1 is most likely polyprotic
(D) suggest serious problems with the pH meter
_________________________________________________________________
5. An experiment was performed to determine the moles of hydrogen gas formed (collected over water) when an acid reacts with magnesium metal. To do this, a piece of dry magnesium was weighed. Then 50 mL of hydrogen were collected. Next the Mg was dried to remove about 0.1 mL of water and weighed again to see how much Mg had reacted. The volume of hydrogen was measured and converted into moles of hydrogen. Which mistake will give the largest error in the result?
(A) Forgetting to dry the magnesium before both weighings
(B) Failing to take the vapor pressure of water (23 torr at 25 °C) into account
(C) Failing to convert °C to K
(D) Reading the gas-collecting container to ±20 mL
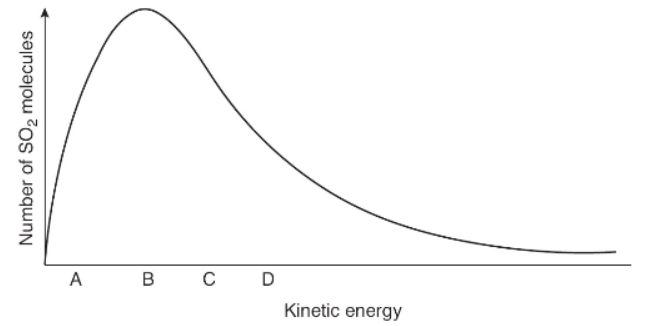
6. The graph above shows the distribution of kinetic energies of a system containing a large number of SO2 molecules at 300 K. Which letter corresponds to molecules having the largest velocity in this system?
(A) A
(B) B
(C) C
(D) D
7. A 25 g sample of a liquid was heated to 95 °C and then quickly transferred to an insulated container holding 100 g of water at 26 °C. The temperature of the mixture rose to reach a final temperature of 37 °C. Which of the following can be concluded?
(A) The sample lost more heat energy than the water gained because the sample temperature changed more than the water temperature did.
(B) The final temperature is less than the average starting temperatures; therefore, the equilibrium constant must be less than 1.
(C) The sample temperature changed more than the water temperature did; therefore, the heat capacity of the sample must be greater than the heat capacity of the water.
(D) Even though the sample temperature changed more than the water temperature did, the sample lost the same amount of heat energy as the water gained to obey the law of conservation of energy.
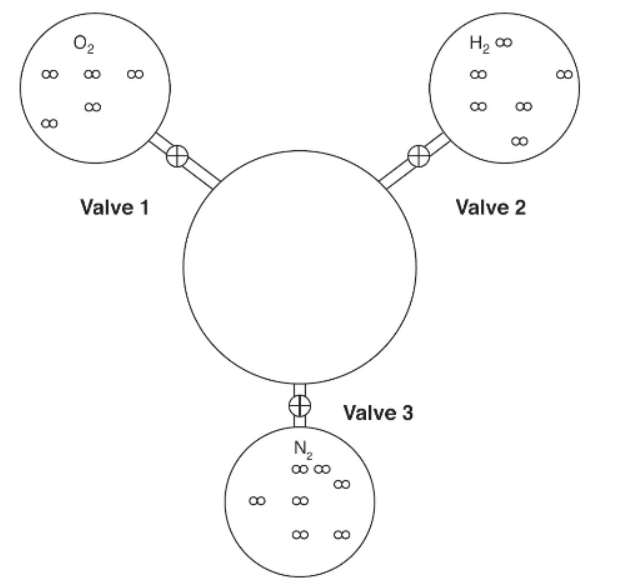
8. Three 1-liter flasks are connected to a 3-liter flask by valves. The 3-liter flask is evacuated to start, and the entire system is at 585 K. The first flask contains oxygen, the second hydrogen, and the third nitrogen. The pressure of hydrogen is 1.65 atm. The amounts of gas molecules are proportional to their representations in the flasks. If valve 2 is opened first and then the rest of the valves are opened, what will the pressure in the 3-liter flask be after the first valve is opened and after they all are opened? Assume the connections have negligible volume.
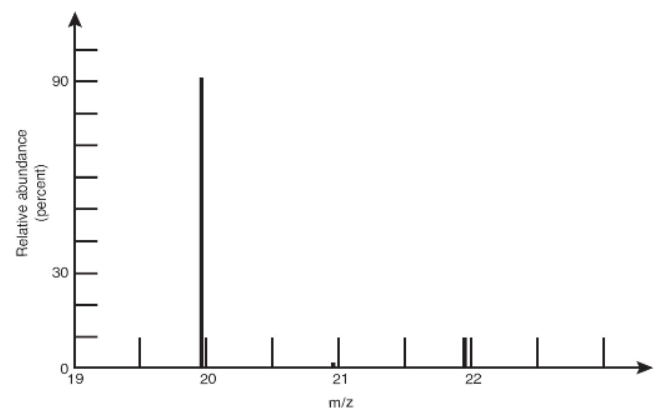
9. A mass spectrum of a naturally occurring sample of an element is shown above. What is the element?
(A) Ca
(B) Ne
(C) K
(D) Not enough information is provided.
Questions 10–13 refer to the titration curve below, which is of a weak base titrated with a strong acid.
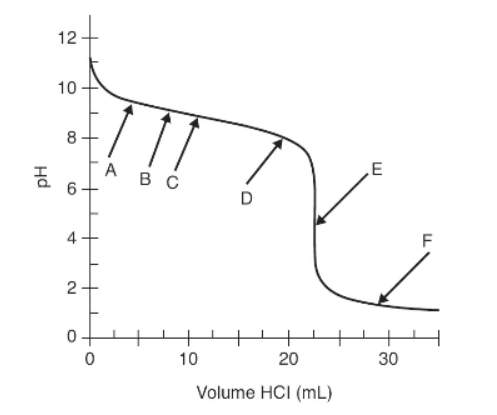
The pH was measured with a pH meter after small volumes of 0.125 M HCl were added to 25.0 mL of a weak base. Data from that experiment are shown in the above graph.
10. What is the molarity of the weak base?
(A) (0.125 M)/((22.0 mL)(25.0 mL))
(B) (0.1 M)(25 mL)/(22 mL)
(C) (25.00 mL)/(0.125 M)(22.5 mL)
(D) (22.5 mL)(0.125 M)/(25.00 mL)
11. Which letter indicates the point closest to the end point of this titration?
(A) Point C because the buffer strength is the highest
(B) Point F because that is the lowest pH given
(C) Point E because it is at the inflection point
(D) Between points A and D for the buffer region
12. At what point and how is the pK for the analyte determined?
(A) At point E, the pK = 7.00
(B) At point C, convert the pH to pOH and that pOH = pKb
(C) At point E, the pKa = pKb
(D) At point C, the pH = pKa
13. Why isn’t the end point at pH 7.00?
(A) Extra acid is needed to neutralize the base.
(B) The cation of a weak base is the major ion present, and it is a weak acid.
(C) The person drawing the curve made an error, and all end points are at pH = 7.00.
(D) The sharp drop at the start pushes the end point downward.
__________________________________________________________________________
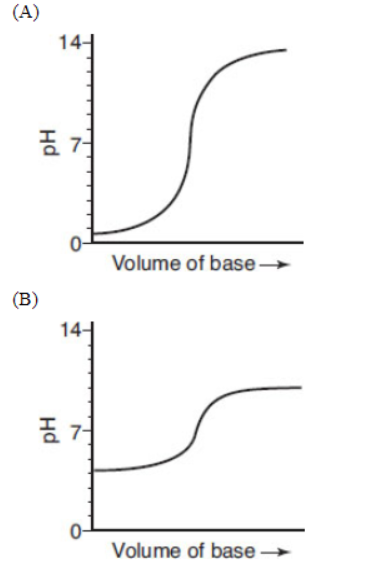
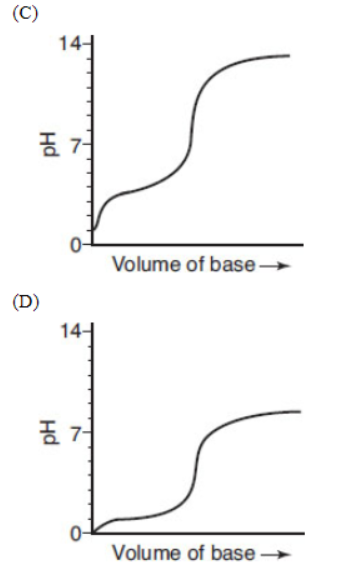
14. A student is performing a titration of a weak acid (0.10 mol L-s1) with a strong base of the same concentration. Which of the following is the best representation of the resulting titration curve?
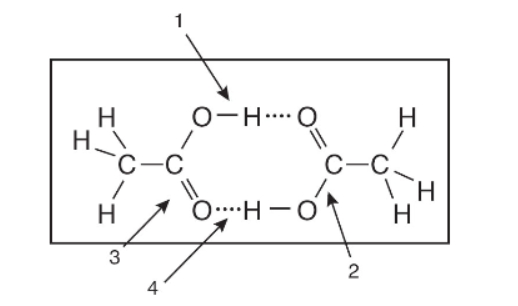
15. In the diagram above, which labeled arrow is pointing toward a covalent bond and which is pointing toward a hydrogen bond?
16. Which would be the easiest way to burn a copper penny?
(A) Hold the copper penny with crucible tongs, and heat strongly in the flame of a Bunsen burner.
(B) Use the method in (A), but use an oxyacetylene torch to reach a higher temperature.
(C) Grind the copper penny into very small, dust-sized particles, and spray the particles into a Bunsen burner flame.
(D) Dissolve the copper penny in acid, precipitate the hydroxide, and heat in a Bunsen burner flame to make the oxide.
S(s) + O2(g) → SO2(g)
17. The equation for the reaction between sulfur and oxygen, written in equation form above, can be interpreted in all of the following ways EXCEPT
(A) one atom of S reacts with one molecule of O2 to yield one molecule of SO2
(B) one mole of sulfur atoms reacts with one mole of oxygen molecules to yield one mole of sulfur dioxide molecules
(C) this reaction goes to completion
(D) adding S(s) will change the equilibrium constant
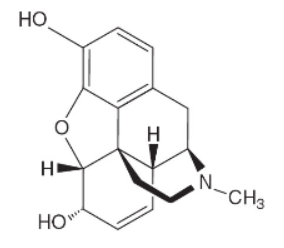
18. Morphine, C17H19N3 (shown above), has a Kb = 8.0 × 10-7. If a 0.00100 M solution of morphine is prepared, the expected pH will be in which one of the following pH ranges?
(A) 2 to 4
(B) 4 to 6
(C) 8 to 10
(D) 10 to 12
19. What is the pKa of morphine hydrochloride, 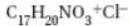 , where
, where .png) is the conjugate acid of the morphine in the previous question?
is the conjugate acid of the morphine in the previous question?
(A) log(8.0 × 10-7)
(B) -log(8.0 × 10-7)
(C) log(1.0 × 10-14/8.0 × 10-7)
(D) -log(1.0 × 10-14/8.0 × 10-7)
Questions 20–23 refer to the following table of information.
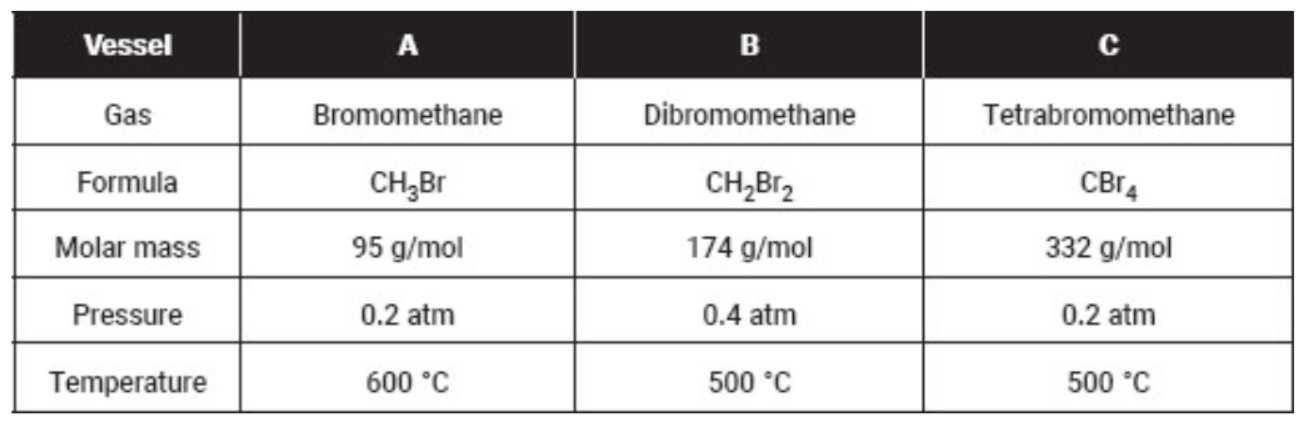
Note that the gases are held in separate, identical, rigid vessels.
20. Which sample has the lowest density?
(A) All have the same density since the temperatures are all the same.
(B) Vessel A
(C) Vessel B
(D) Vessel C
21. The average kinetic energy
(A) is greatest in vessel A
(B) is greatest in vessel B
(C) is greatest in vessel C
(D) is the same in all vessels
22. Which of these gases is expected to condense at the lowest pressure, assuming that the temperature is held constant?
(A) Bromomethane
(B) Dibromomethane
(C) Tetrabromomethane
(D) They all condense at the same pressure.
23. Which attractive force is the major cause of condensation?
(A) Hydrogen bonding
(B) London forces
(C) Dipole-dipole attractive forces
(D) Molar mass
___________________________________________________________________

24. Use the octet rule without minimizing formal charges and the arrangement of atoms suggested in the skeleton structure above to construct the Lewis structure for the SO3 molecule. All of the following statements about this molecule are true EXCEPT
(A) sulfur trioxide has three resonance structures
(B) sulfur trioxide has a planar triangle shape
(C) sulfur trioxide is nonpolar
(D) the S-O bond order is 5/3
.png)
25. Use the octet rule and the arrangement of atoms suggested in the skeleton structure above to construct the Lewis structure for the sulfite ion. Be sure to minimize the formal charges. All of the following statements are true EXCEPT
(A) the sulfite ion has a double bond
(B) the sulfite ion has a triangular pyramid shape
(C) the sulfite ion is nonpolar
(D) the S-O bond order is 4/3
Questions 26–28 refer to the following information.
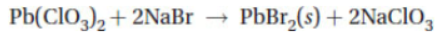
A chemist mixes a dilute solution of lead chlorate with a dilute solution of sodium bromide to precipitate lead(II) bromide (Ksp = 4.0 × 10-5).
26. Which of the following is the net ionic equation for the experiment described above?
(A)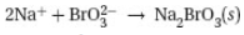
(B) 2Br- + Pb2+ → PbBr2(s)
(C) 4Br- + Pb4+ → PbBr4(s)
(D)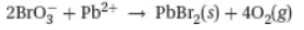
27. If equal volumes of 2.0 millimolar solutions are mixed, which of the following particulate views represents the experiment after the reactants are mixed thoroughly and the solids are given time to precipitate?
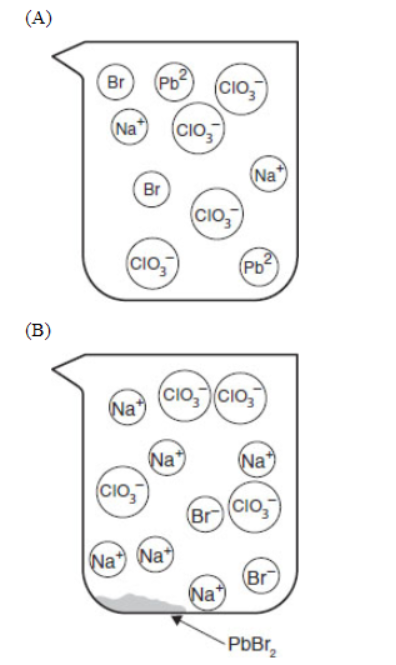
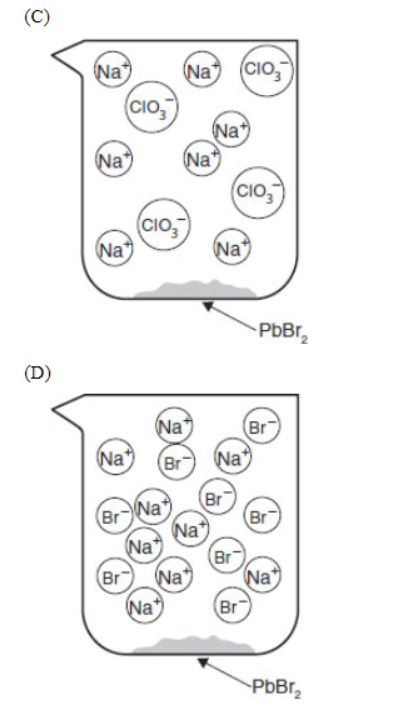
28. Within a factor of 10, what is the approximate molar solubility of PbBr2 in a solution of 0.17 M Pb2+ ions?
(A) 7.7 × 10-15M
(B) 7.7 × 10-8M
(C) 4.0 × 10-5M
(D) 7.7 × 10-3M
Questions 29–33 refer to the information and diagram below.
N2O4(g) ⇌ 2NO2(g)
N2O4(g) decomposes into NO2(g) according to the equation above. A pure sample of N2O4(g) is placed into a rigid, evacuated 0.500 L container. The initial pressure of the N2O4(g) is 760 torr. The temperature is held constant until the N2O4(g) reaches equilibrium with its decomposition products. The figure below shows how the pressure of the system changes while reaching equilibrium.
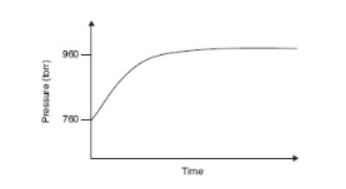
29. Which is a correct description of the partial pressures of N2O4 and NO2?
(A) The partial pressures of both N2O4 and NO2 are increasing.
(B) The partial pressures of both N2O4 and NO2 are decreasing.
(C) The partial pressure of N2O4 is increasing twice as fast as that of NO2 is decreasing.
(D) The partial pressure of NO2 is increasing twice as fast as that of N2O4 is decreasing.
30. The pressure versus time curve can be used to evaluate
(A) the kinetics of the reaction from the rising part and the equilibrium constant from the horizontal part
(B) only the kinetics of the reaction from the rising part
(C) only the equilibrium constant from the horizontal part
(D) only the value of Q
31. What will the final pressure be if this reaction goes to completion? Based on the data, does this reaction go to completion?
(A) 760 torr; yes, the reaction goes to completion because this is the given pressure.
(B) 900 torr; yes, the reaction goes to completion because it reaches equilibrium.
(C) 1520 torr; no, the reaction does not go to completion because it is not 1520 torr when the reaction stops.
(D) 2280 torr; no, the reaction does not go to completion because it is not 2280 torr when the reaction stops.
32. What is the appropriate combination of numbers for calculating Kp?

33. Which diagram is an appropriate description of the system if more NO2(g) is rapidly injected, at time = 0, into the container after equilibrium is established?
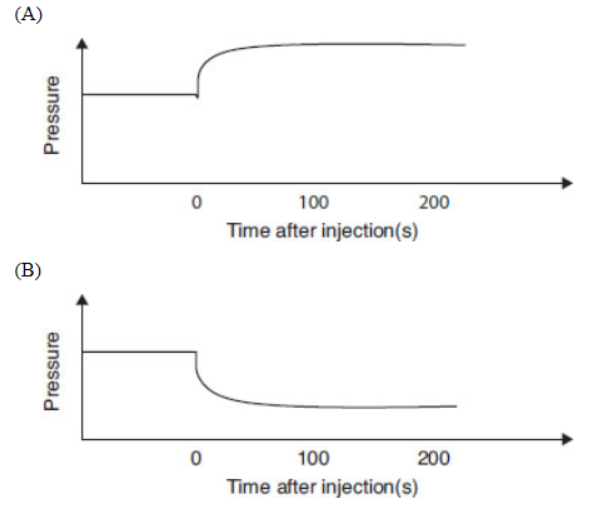
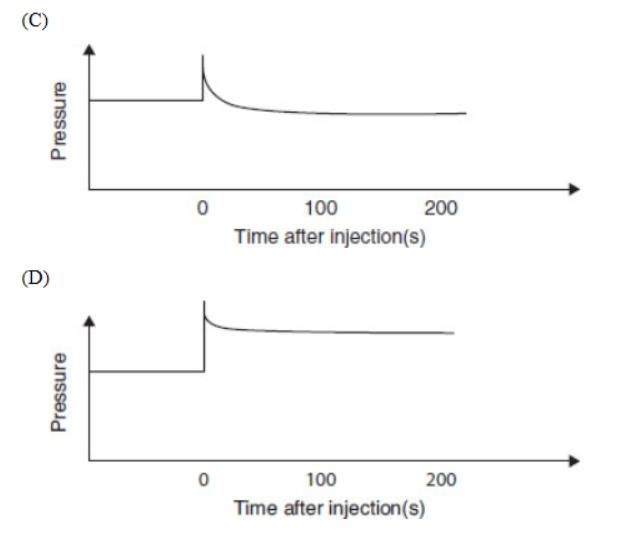
Formation of a solution can often be visualized as a three-step process.
Step 1. Solvent molecules are separated from each other to make space for the solute.
Step 2. Solute molecules are separated so they fit into spaces in the solvent.
Step 3. Separated solute and solvent are brought together filling in the spaces.
34. All of the fundamental principles below are important in understanding the formation of solutions EXCEPT which one?
(A) Starting at equilibrium, moving particles apart while in the solid or equilibrium phases requires added energy proportional to the attractive forces.
(B) Bringing particles together releases energy in proportion to their attractive forces.
(C) The total of the energies in steps 1 to 3 indicates if a solution will form.
(D) Molecules with similar molecular masses are required to form solutions.
35. Bromine has a normal boiling point of 59 °C, and iodine boils at 184 °C. The I2 molecule is much larger than Br2 (atomic radii are 114 and 133 pm, respectively). Which is the best reason for the large difference in boiling points?
(A) Bromine is a liquid and boils; iodine is a solid and sublimes.
(B) The intramolecular bonds in I2 are much weaker than those in Br2.
(C) The I2 electron clouds are much more polarizable than the Br2 electron clouds, resulting in much stronger London forces.
(D) The mass of iodine is much greater than the mass of bromine.
36. Ethanoic acid (HC2H3O2 or CH3CO2H or CH3COOH) has a much lower vapor pressure than ethanol (CH3CH2OH). What is the most reasonable explanation?
(A) The polarizability of two oxygen atoms increases the London forces of attraction in ethanoic acid compared with ethanol.
(B) Hydrogen bonding in ethanoic acid is the strongest attractive force and is mainly responsible for the observed data.
(C) Ethanol has an -OH group and can hydrogen bond; therefore, the London forces must cause the effect.
(D) Both ethanol and ethanoic acid have an -OH, so the difference is the dipole of the second oxygen that increases the attractive forces.
37. Which reaction is expected to be endothermic?
(A) 2CO(g) + O2(g) → 2CO2(g)
(B) 2C6H6(l) + 15O2(g) → 12CO2(g) + 6H2O(g)
(C) 2C6H6(l) → 2C6H6(g)
(D) F2(g) +2e- → 2F-(g)
38. Which of the following is a formation reaction?
(A) MnO4- + 8H+ + 5e- → Mn2+ + 4H2O
(B) 2CO(g) + O2(g) → 2CO2(g)
(C) 2O2(g) + N2(g) → 2NO2(g)
(D)
39. Which method cannot be used to determine the enthalpy change of a chemical reaction?
(A) Measure the entropy change for the reaction at 298 K.
(B) Combine the heats of reaction of appropriate reactions using Hess’s law.
(C) Use the necessary heats of formation to calculate the heat of reaction.
(D) Use a calorimeter to determine the heat absorbed or evolved in the reaction.
Questions 40–41 refer to the following thermochemical reactions.

40. What is the enthalpy of the reaction N2 + 2O2 → 2NO2?
(A) −294.0 kJ
(B) −67.6 kJ
(C) +294.0 kJ
(D) The enthalpy of the reaction cannot be determined with this information.
41. How much heat is produced or absorbed if 0.200 mol N2 is reacted with 0.300 mol O2 to form NO?
(A) 54.2 kJ of heat is absorbed.
(B) 58.8 kJ of heat is evolved.
(C) 22.6 kJ of heat is evolved.
(D) 36.2 kJ of heat is evolved.
Questions 42–45 refer to the cell diagrams and their corresponding 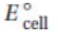 values for the three galvanic cells listed in the table below.
values for the three galvanic cells listed in the table below.
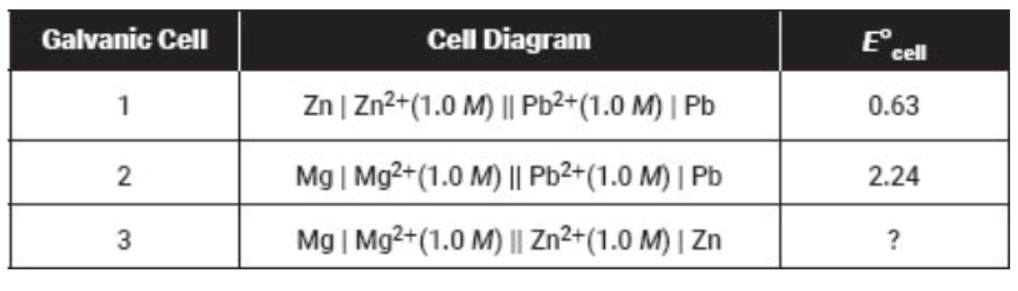
The chemical reaction occurring in the first galvanic cell is
Zn + Pb2+ → Zn2+ + Pb
42. What is the standard cell potential for galvanic cell 3 depicted in the table above?
(A) 2.77 V
(B) 1.61 V
(C) 0.18 V
(D) −1.61 V
43. What is the chemical reaction under study in galvanic cell 3?
(A) Pb2+ + Mg2+ → Pb2+ + Mg
(B) Zn + Pb2+ → Zn2+ + Pb
(C) Mg + Zn2+ → Mg2+ + Zn
(D) Zn + Mg2+ → Zn2+ + Mg
44. If the concentration of Mg2+ is changed from 1.0 M to 0.1 M in the galvanic cells above, what will happen to the observed cell voltages in galvanic cells 2 and 3?
(A) The voltage in both galvanic cells 2 and 3 will increase.
(B) The voltage will decrease in both galvanic cells 2 and 3.
(C) The voltage in galvanic cell 2 will increase and will decrease in galvanic cell 3.
(D) The voltage in galvanic cell 3 will increase and will decrease in galvanic cell 2.
45. Rank Mg, Pb, and Zn from the metal that is easiest to oxidize to the metal that is the most difficult to oxidize.
(A) Mg, Pb, Zn
(B) Zn, Pb, Mg
(C) Mg, Zn, Pb
(D) Pb, Zn, Mg
Carbon has an atomic radius of 77 pm and a first ionization energy of 1086 kJ/mol.
46. Based on periodic trends and the data given above, what are the most probable values for the atomic radius and first ionization energy of nitrogen?
(A) 70 pm, 1402 kJ/mol
(B) 86 pm, 898 kJ/mol
(C) 135 pm, 523 kJ/mol
(D) 40 pm, 995 kJ/mol
47. Dissolving one mole of each of the following compounds in water results in solutions with different pH values. Under those conditions, which of the following acids will have the highest percentage ionization
(A) HNO2
(B) HClO4
(C) H2S
(D) H3PO4
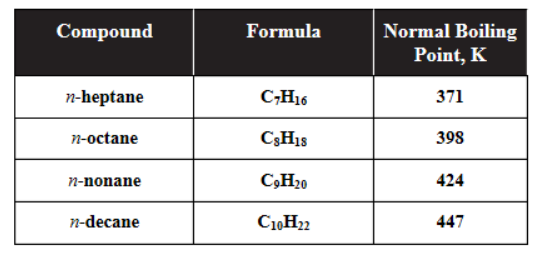
48. Based on the data in the table above, which of the following substances has the lowest viscosity?
(A) n-heptane
(B) n-octane
(C) n-nonane
(D) n-decane
49. Based on periodic relationships, the concepts related to bond strength, and the concept relating bond strength to acid strength, which of the following correctly predicts the strength of binary acids from strongest to weakest?
(A) H2Se > H2O > H2S
(B) H2S > H2Se > H2O
(C) H2O > H2S > H2Se
(D) H2Se > H2S > H2O
50. Chlorine is often used to oxidize other substances. It makes a good bleach because it can oxidize many colored compounds. If chlorine is not available, what other substance can be used for oxidizing substances?
(A) Al
(B) H2S
(C) Zn
(D) KMnO4
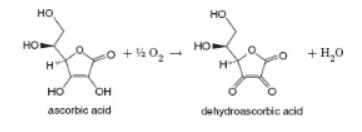
Vitamin C is oxidized slowly to dehydroascorbic acid by the oxygen in air. It is catalyzed by ions such as Cu2+ and Fe3+. The reaction can be followed by measuring the ultraviolet absorbance at 243 nm.
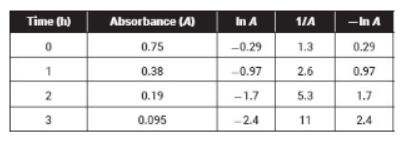
51. Which of the following is the best interpretation of the data given above?
(A) The reaction is first order, and the rate constant cannot be calculated from these data.
(B) The reaction is zero order, and the rate constant can be calculated from these data.
(C) The reaction is first order, and the rate constant can be calculated from these data.
(D) The reaction is second order, and the rate constant cannot be calculated from these data.
52. A new compound is synthesized and found to be a monoprotic acid with a molar mass of 248 g/mol. When 0.0050 mol of this acid is dissolved in 0.500 L of water, the pH is measured as 3.89. What is the pKa of this acid?
(A) 2.33
(B) 3.89
(C) 5.78
(D) 7.78
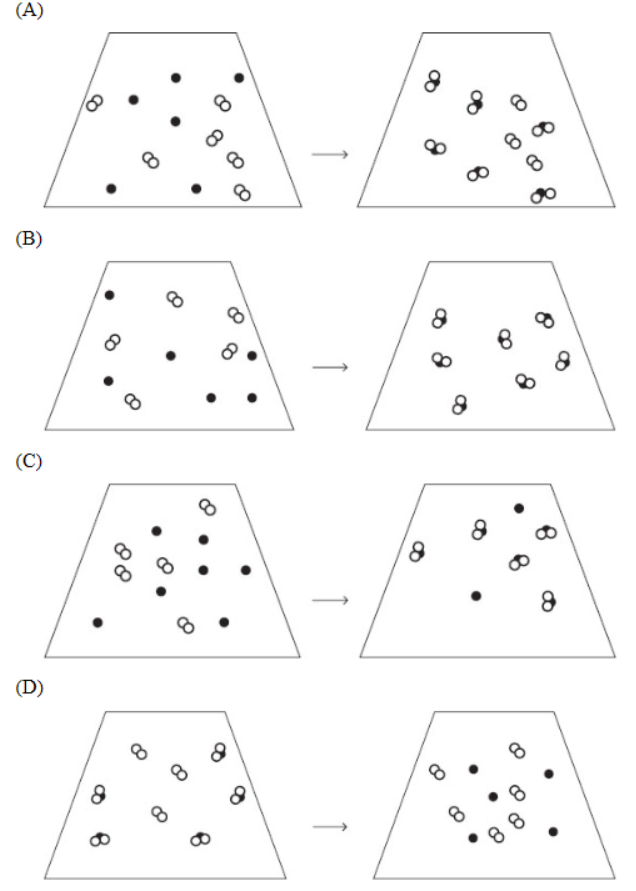
53. Which of the following particulate diagrams best represents the reaction of gaseous sulfur (black dots) with oxygen (white circles) to form sulfur dioxide?
54. Nitrous acid is a weak acid, while nitric acid is a much stronger acid because
(A) the nitrogen in nitric acid is more electronegative than the nitrogen in nitrous acid
(B) nitric acid has more oxygen atoms in its formula
(C) the -O-H bonds in nitric acid are much weaker than in nitrous acid due to the electron withdrawing of more oxygen atoms on nitric acid
(D) nitric acid has the hydrogen atoms bound directly to the nitrogen atom
55. A student has a liter of a 0.100 M solution of a strong acid. To prepare a buffer, this should be mixed with
(A) a strong acid
(B) a weak acid
(C) a weak base
(D) a strong base
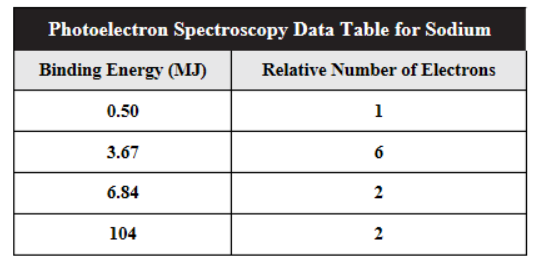
56. The photoelectron data for sodium are shown above. Which of the following statements is true about this data?
(A) All 11 electrons are shown in the table.
(B) There are 9 valence electrons.
(C) The 2s electrons have binding energies of energy of 104 MJ/mol.
(D) Peaks with the lowest energies are due to electrons closest to the nucleus.
57. Identify the Brønsted-Lowry conjugate acid–base pair in the following list.
(A) H3O+ and OH-
(B) H3PO4 and H3PO3
(C) HC2H3O2 and C2H3O2-
(D)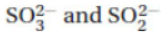
58. Chemical reactions can be classified as either heterogeneous or homogeneous. Which of the following equations below is best classified as a heterogeneous reaction?
(A) 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(g)
(B) 2SO2(g) + O2(g) → 2SO3(g)
(C) C2H2(g) + 5N2O(g) → CO2(g) + H2O(g) + 5N2(g)
(D) C(s) + H2O(g) → H2(g) + CO(g)
59. Which of the reactions below will not be thermodynamically favored at all temperatures?
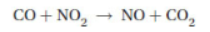
60. Consider the following possible mechanism for this gas phase reaction above:
Step 1. NO2 + NO2 → NO3 + NO rate-limiting step
Step 2. NO3 + CO → NO2 + CO2
Which of the following statements is not true?
(A) NO3(g) is a catalyst for this reaction.
(B) The rate law for this mechanism is Rate = k[NO2]2.
(C) NO3(g) is an intermediate.
(D) The mechanism adds up to the overall reaction.
Section II: Free-Response
Start only when you have 105 minutes to complete the whole section.

Instructions
The questions appear on the following pages. You may use the periodic table and the table of equations and constants that were provided with Section I. On the actual exam, you will be directed to write your final answers only in the test booklet. For this practice test, write your answers on separate sheets of lined paper. You will need about three pages for each of questions 1 through 3 and one page for each of questions 4 through 7.
Do not spend too much time on any one question. Budget your time carefully, and answer the easier questions first.
Be sure that your work is well organized, complete, and easy to read. Cross out or erase any mistakes. Erased and crossed-out material will not be scored.
Section II
7 Free-Response Questions
(Time: 105 minutes)
CALCULATORS ARE ALLOWED FOR SECTION II

1. A student is comparing the slightly soluble CaCO3 and PbCO3 to understand their solubility properties.
(a) Write the Ksp equations for CaCO3 and PbCO3 in water.
(b) The student had a conductivity meter to make measurements on solutions of CaCO3 and PbCO3. Should the student compare two solutions that have the same mass per liter or should moles per liter be examined? Explain your answer.
(c) The student tries an experiment where two 500 mL solutions are made and each contains the same number of moles of solute. One solution contains CaCO3, and the other contains PbCO3. A small amount of solid is observed on the bottom of both beakers. What should the student observe on the conductivity meter?
The student then added the same number of moles of nitric acid to each solution in part (c) until one precipitate completely disappeared.
(d) Which solid disappeared? Justify your reasoning.
(e) In very old water supplies in old cities, the pipes have developed an inner coating of CaCO3 over many years. In some places, PbCO3 can be found along with CaCO3. Suggest why it is very important to control the pH of the public water supply in this situation.
(f) Ocean beaches in the northern and southern latitudes of Earth have “sand” that is most often silicon dioxide (SiO2). In latitudes nearer the equator, the “sand” in ocean beaches tends to be composed of calcium carbonate. Suggest a reasonable explanation for the two types of sand.
2. A solid organic compound was extracted from hops (used to make beer) and tobacco.
(a) It was found that the compound was completely soluble in water and the molecular mass was determined to be 102 g/mol. When the solution of the compound was tested using a flame test, it indicated no presence of any common metals. Therefore, the likely elements in the formula for the compound either are C and H or are C, H, and O. Explain how a flame test works.
(b) Another 1.234 g sample of the compound was placed into an apparatus devised to burn the compound completely. The carbon dioxide formed in the combustion was absorbed in one tube, and the water formed was collected by an absorbent. The results were that 2.662 g of CO2 were collected. Also, 1.089 g of H2O was collected. How many grams of carbon and hydrogen were collected?
(c) If the masses of carbon and hydrogen in part (d) add up to the 1.234 g (+/-0.1 g) of sample burned, it means that there are no other elements in the chemical formula. If the masses add to less than 1.234 g, it is likely that oxygen is present. Determine how many grams of oxygen are in this compound.
(d) Calculate the moles of C, H, and O in this compound.
(e) When converted to integers, how many moles of C, H, and O are in the formula for this compound? What is the empirical formula?
(f) Use the molecular mass and empirical formula to determine the molecular formula.
3. Trimethylamine, (CH3)3N, ionizes when dissolved in water according to the equation below.
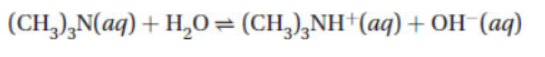
(a) Write the expression for the equilibrium constant, Kb, for the reaction.
(b) Calculate the pOH of a 0.036 M solution of trimethylamine.
The value of Kb = 7.4 × 10-5
The chemical equation below is the balanced equation for the ionization of dichloroacetic acid, Cl2CHCOOH, in water.
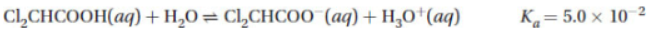
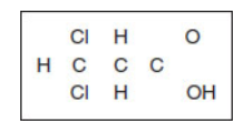
(c) The atoms in dichloroacetic acid are shown in the box below. They are arranged in the correct positions. Complete the Lewis structure for dichloroacetic acid by including all 44 bonding and nonbonding valence electrons where they belong.
(d) Write the balanced net ionic equation representing the mixing of aqueous solutions of trimethylamine with dichloroacetic acid.
(e) When 55.0 mL of 0.0200 M dichloroacetic acid is mixed with 110.0 mL of 0.0100 M trimethylamine, will the resulting solution be acidic or basic? Justify your answer.
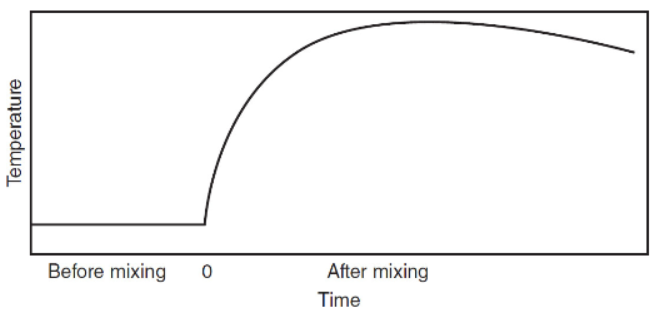
(f) The experiment in part (e) is repeated with a “coffee-cup” calorimeter that minimizes heat loss. Sketch a diagram that is a plot of temperature vs. time based on a digital thermometer continuously measuring the temperature. Describe the best way to determine the temperature change that represents the heat of neutralization of this acid and base.
(g) Write the overall reaction and the equilibrium expression that is occurring in parts (e) and (f).
(h) Does dichloroacetic acid have two or more resonance structures? Explain.
(i) Is dichloroacetic acid expected to be stronger or weaker than acetic acid? Explain your answer.
4. The basic values of thermodynamics are the enthalpy of a reaction, the entropy of a reaction, and the free-energy change of a reaction.
(a) What is the fundamental measuring device used to determine the standard enthalpy of a reaction, ΔH°reaction? How is that device used?
(b) What is the fundamental measuring device used to determine the standard free-energy change, ΔG°reaction, for a chemical reaction? How is that device used to determine ΔG°reaction?
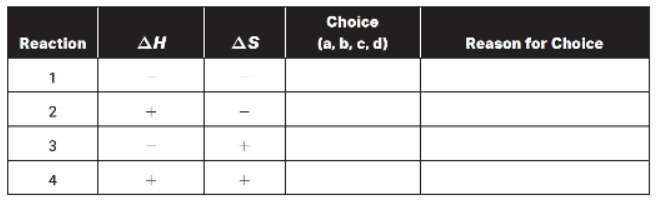
(c) Fill in the following table. Use the letters shown to indicate the type of reaction:
(a) always spontaneous
(b) always not spontaneous
(c) spontaneous at low temperatures and nonspontaneous at high temperatures
(d) spontaneous at high temperatures and nonspontaneous at low temperatures
(d) Write the balanced formation equation for ammonium phosphate (NH3)3PO4.
5. Elements that are radioactive do not have stable nuclii. Some emissions of radioactive elements are beta particles, alpha particles, and gamma rays.
(a) Briefly describe each of these emissions. Include the symbol and its relative energy.
(i) Alpha particles
(ii) Beta particles
(iii) Gamma rays
(b) Determining the age of archaeological finds that once were alive often uses carbon-14 (also called 14C). Describe the process of dating using 14C.
(i) Since 14C is not a naturally occurring isotope on Earth, how and where is it produced?
(ii) The ratio of 14C to 12C is measured in carbon-14 dating. What basic assumption about 14C is made about this isotope on Earth?
(c) The half-life of 14C is 5730 years.
(i) Kinetically, what is the order of radioactive decay for 14C?
(ii) What is the rate constant for the decay of 14C in units of years-1?
6. Lewis electron-dot structures are often used to understand and compare the bonding in different molecules.
(a) Draw the Lewis electron-dot structures for carbon monoxide (CO), carbon disulfide (CS2), and carbon tetrachloride (CCl4).
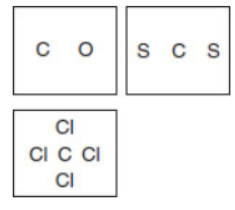
(b) Which compound has a tetrahedral shape: carbon monoxide (CO), carbon disulfide (CS2), or carbon tetrachloride (CCl4)? Justify your answer.
(c) In part (a), assign the hybridization to each bond for carbon monoxide (CO), carbon disulfide (CS2), and carbon tetrachloride (CCl4).
7. Hydrogen iodide is a gas at room temperature (298 K), and the reaction to make HI is
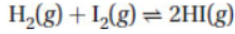
The following table contains all thermodynamic data for the reaction above:
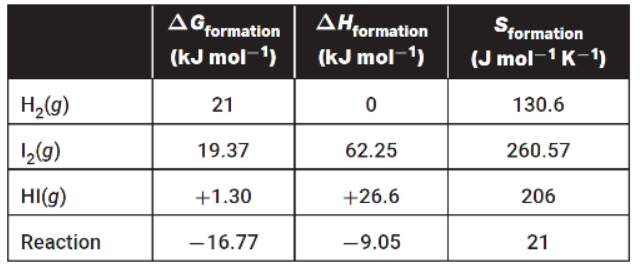
(a) Three balloons are filled with gases. One is filled with hydrogen, one with iodine, and one with hydrogen iodide. All three balloons are at room temperature and have 1 atmosphere of pressure. When released, which balloon(s) will rise to the ceiling and which will drop to the floor? Justify your answer.
(b) When the substances are combined and are placed into a rigid container such as a sealed flask and the pressure is measured as 560 torr, will the pressure change if the temperature is increased by 15 K?
(c) What are the two differences between ideal gases and real gases?
(d) Why does adding gas atoms (or molecules) to a rigid container raise the pressure (one reason)? Why does an increased temperature raise the pressure of a gas in a rigid container (two reasons)?




