Practice Test 1 - AP Chemistry Premium 2024
Section I: Multiple-Choice
Start only when you have 90 minutes to complete the whole section.
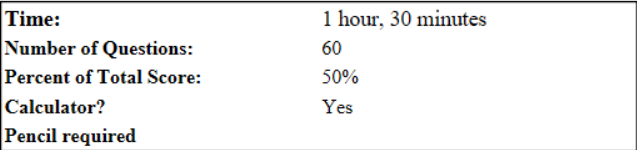
Instructions
There are 60 multiple-choice questions for this part of the exam. Enter your answers on the answer sheet provided. On the actual exam, no credit will be given for answers marked on the test itself. For this test, mark your selected answer next to the question and then transfer it to the scoring sheet. (This will allow you to check that all answers are properly transferred to the scoring sheet.) Each question has only one answer. When changing answers, be sure to erase completely.
Useful Hints
Not everyone will know the answers to all of the questions. However, it is to your advantage to provide an answer to all questions. Do not waste time on difficult questions. Answer the easier ones first, and return to the difficult ones you have not answered if time remains.
Your total score is simply the number of questions answered correctly. Wrong answers or blanks on the scoring sheet do not count against you.
You will be allowed to use the following periodic table and the table of equations and constants on this part of the test.
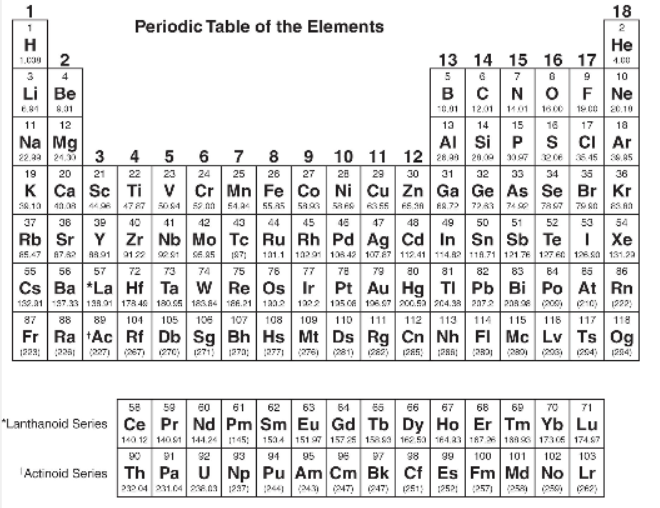
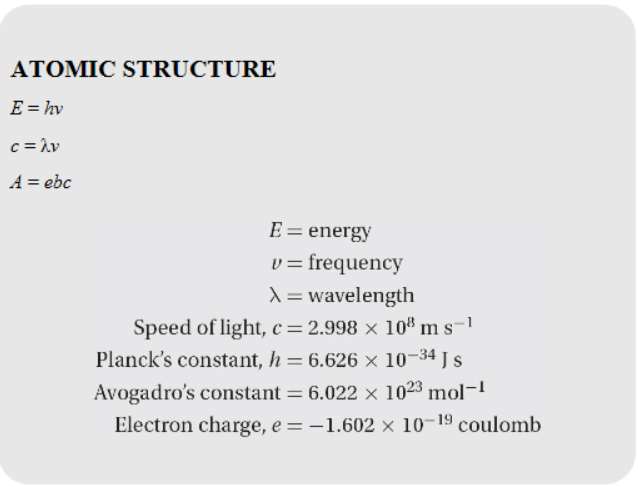
Equations and Constants
GENERAL INFORMATION
Section I
60 Multiple-Choice Questions
(Time: 90 minutes)
CALCULATORS ARE ALLOWED FOR SECTION I
Note: For all questions, assume that T = 298 K, P = 1.00 atmosphere, and H2O is the solvent for all solutions unless the problem indicates a different solvent.

1. Using fundamental trends in electronegativity and bond strength, which of the following should be the strongest acid?
(A) H2S because it is the heaviest
(B) HI because the bond length is longest
(C) HBr because Br has the highest electronegativity
(D) H2O because water forms the strongest hydrogen bonds
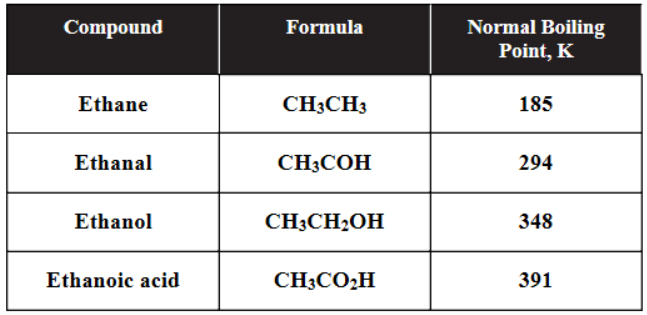
2. Based on the data in the table above, which of the following substances has the lowest surface tension at -120 °C?
(A) Ethane since it doesn’t form hydrogen bonds
(B) Ethanal since its hydrogen bonds are the weakest
(C) Ethanol since strong hydrogen bonds reduce surface tension
(D) Ethanoic acid because it is the heaviest molecule
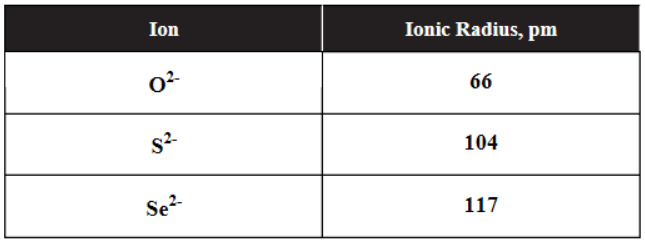
3. Based on the data in the table above, which of the following correctly predicts the strength of binary acids from weakest to strongest?
(A) H2S < H2O < H2Se
(B) H2Se < H2O < H2S
(C) H2S < H2Se < H2O
(D) H2O < H2S < H2Se
4. Zinc metal is often used to reduce substances in aqueous solutions. If zinc metal is not available, which of the following would be a reasonable and safe substitute for zinc?
(A) S
(B) F2
(C) K
(D) Cd
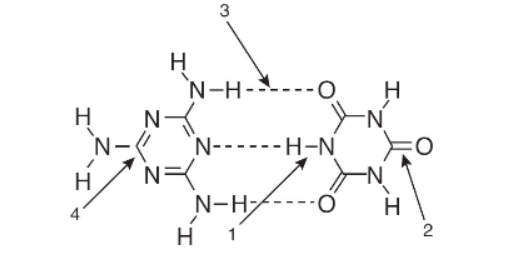
5. In the diagram above, which labeled arrow is pointing toward a hydrogen bond?
(A) 1
(B) 2
(C) 3
(D) 4
6. Which would be the easiest way to burn an iron nail?
(A) Hold an iron nail with crucible tongs, and heat strongly in the flame of a Bunsen burner.
(B) Use the method in (A), but use an oxyacetylene torch to reach a higher temperature.
(C) Grind the nail into very small, dust-sized particles, and spray them into a flame.
(D) Dissolve the nail in acid to make the oxide.
S(s) + O2(g) → SO2(g)
7. The reaction of sulfur with oxygen is written in equation form above. This equation can be interpreted in all of the following ways EXCEPT
(A) either S(s) or O2(g) will be completely used up
(B) Q must be close to 1.0 since there is one mole of gas on each side of the equation
(C) this reaction goes to completion
(D) adding O2 will not change the equilibrium constant
8. An experiment was performed to determine the moles of hydrogen gas formed (collected over water) when an acid reacts with magnesium metal. To do this, a piece of dry magnesium was weighed. Then 50 mL of hydrogen were collected. Next the Mg was dried to remove approximately 0.1 mL of water and weighed again to see how much Mg had reacted. The atmospheric pressure was measured as 755 torr. The volume of hydrogen was measured and converted into moles of hydrogen. Which mistake will give the smallest error in the result?
(A) Forgetting to dry the magnesium before weighing the second time
(B) Failing to take the vapor pressure of water (23 torr at 25 °C) into account
(C) Failing to convert °C to K
(D) Reading the gas-collecting container to ±20 mL
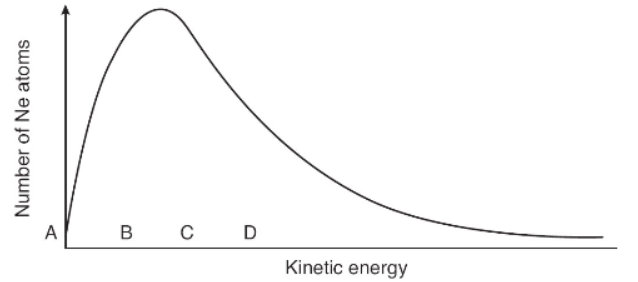
9. The graph above shows the distribution of kinetic energies of a large number of Ne atoms at 500 K. Which letter shows the average kinetic energy of this system?
(A) A
(B) B
(C) C
(D) D
10. A 25 g sample of a liquid was heated to 100 °C and then quickly transferred to an insulated container holding 100 g of water at 22 °C. The temperature of the mixture rose to reach a final temperature of 35 °C. Which of the following can be concluded?
(A) The sample temperature changed more than the water temperature did; therefore, the sample lost more heat energy than the water gained.
(B) The sample temperature changed more than the water temperature did, but the sample lost the same amount of heat energy as the water gained.
(C) The sample temperature changed more than the water temperature did; therefore, the CP of the sample must be greater than the CP of the water.
(D) The final temperature is less than the average starting temperature of the sample and the water; therefore, the total energy of the sample and water decreased.
11. Identify the Brønsted-Lowry conjugate acid–base pair in the following list.
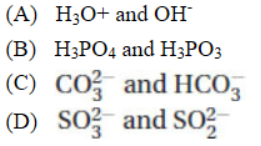
12. Chemical reactions can be classified as either heterogeneous or homogeneous. Which of the following equations below is best classified as a heterogeneous reaction?
(A) 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(g)
(B) C2H5OH(g) + O2(g) → HC2H3O2(g) + H2O(g)
(C) 2Fe(s) + 3CO2(g) → Fe2O3(s) + 3CO(g)
(D) C2H2(g) + 5N2O(g) → CO2(g) + H2O(g) + 5N2(g)
13. Which of the reactions below will be thermodynamically favored at all temperatures?
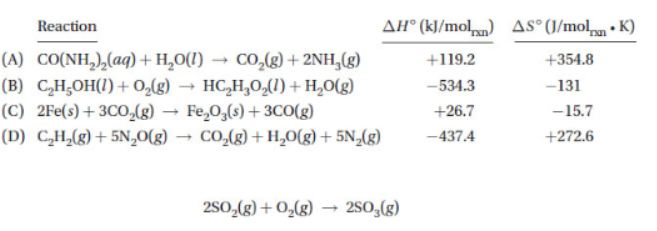
14. Consider the following possible mechanism for the reaction above:
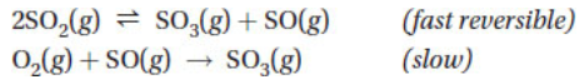
Which of the following statements is true?
(A) This mechanism has no free radicals.
(B) O2(g) is an intermediate.
(C) SO(g) is a catalyst for this reaction.
(D) The mechanism does not add up to the overall reaction.
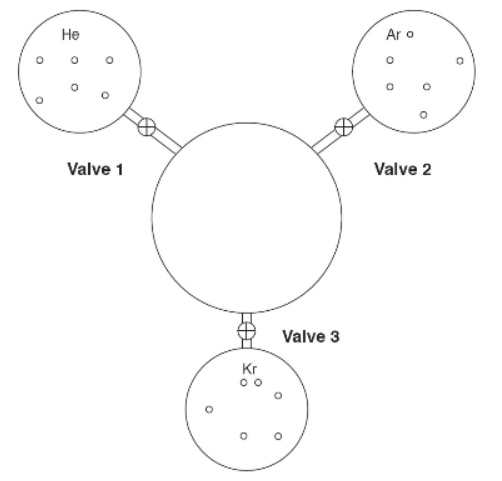
15. Three 1-liter flasks are connected to a 3-liter flask by valves. The 3-liter flask is evacuated to start, and the entire system is at 298 K. The first flask contains helium, the second argon, and the third krypton. The pressure of the argon is 633 torr. The amounts of gas are proportional to their representations in the flasks. If all the valves to the center flask are opened, what will the pressure of the system be? Assume the connections have negligible volume.
(A) 211 torr
(B) 316 torr
(C) 633 torr
(D) 1266 torr
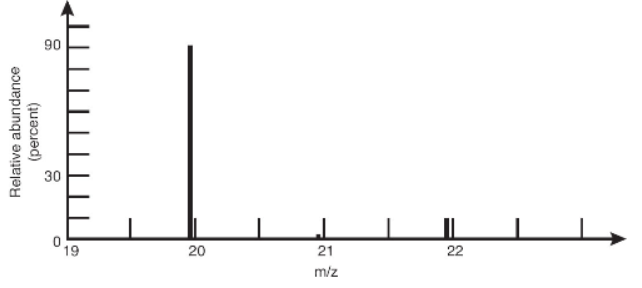
16. A portion of a mass spectrum of neon is presented above. Estimate the average mass of naturally occurring atoms of neon, assuming that the height of each line represents the relative amount of each mass.
(A) 20.0
(B) 20.3
(C) 21.0
(D) Not enough information is provided.
17. Which of the following particulate diagrams best represents the reaction of carbon monoxide with oxygen to form carbon dioxide?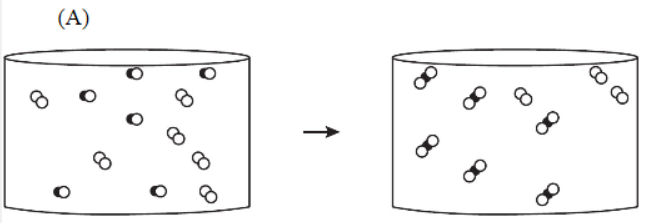
Questions 18–22 refer to the following diagram and data.
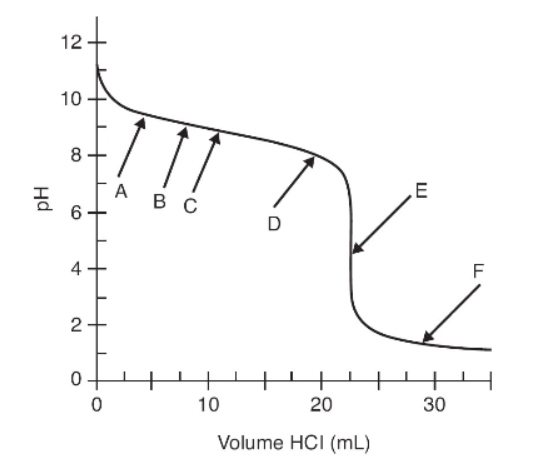
The above graph shows a titration curve of a weak base titrated with a strong acid. The pH was measured with a pH meter after small volumes of 0.075 M HCl were added to 25.0 mL of a weak base. Data from that experiment are shown in the graph.
18. Which arrow points to the end point of this titration?
(A) A
(B) E
(C) C
(D) F
19. Which arrow points to the place on the curve where the pH is equal to 14 - pKb?
(A) A
(B) E
(C) C
(D) F
20. If the student used a pH indicator that changes color from pH 5 to 7, which statement best characterizes the expected observations?
(A) The observed color change will be distinct, and the calculated molarity of the base will be accurate.
(B) The color will change slowly, and the end point volume will be low.
(C) The color will change slowly, and the calculated molarity of the base will be low.
(D) The observed color change will be distinct, but the calculated molarity of the base will be high.
21. Find the two points on the curve that indicate the region where the solution can be described as a buffer. What is the change in pH from the first point to the second?
(A) 0.5 pH unit
(B) 1 pH unit
(C) 2 pH units
(D) 3 pH units
22. Which of the following describes the base that is being titrated?
(A) The base is dibasic since two end points are observed.
(B) The concentration of the base is 0.075 M.
(C) The concentration of the base is slightly more than 0.075 M.
(D) The concentration of the base is slightly less than 0.075 M.
___________________________________________________________
23. Methylamine, CH3NH2, has a Kb = 4.4 × 10-4. If a 0.0100 M solution of methylamine is prepared, the expected pH will be in which one of the following pH ranges?
(A) 2 to 4
(B) 4 to 6
(C) 8 to 10
(D) 10 to 12
24. What is the pKa of the methylammonium ion, 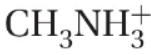 which is the conjugate acid of methylamine described in the previous question?
which is the conjugate acid of methylamine described in the previous question?
(A) log(4.4 × 10-4)
(B) -log(4.4 × 10-4)
(C) -log(4.4 × 10-4/1.0 × 10-14)
(D) log(4.4 × 10-4/1.0 × 10-14)
Questions 25–28 refer to the table below.
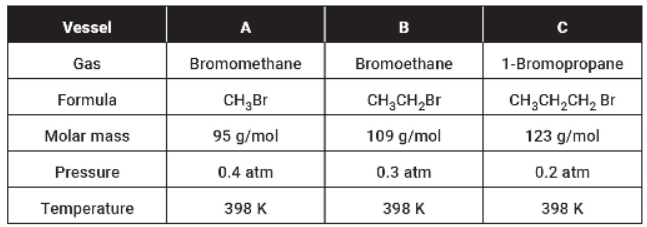
Note that in addition to the data in the table, the gases are held in separate, identical, rigid vessels.
25. The sample with the lowest density is
(A) the same in all three samples since the temperatures are all the same
(B) in vessel A
(C) in vessel B
(D) in vessel C
26. The average kinetic energy
(A) is greatest in vessel A
(B) is greatest in vessel B
(C) is greatest in vessel C
(D) is the same in all vessels
27. Which of these gases will condense at the lowest pressure, assuming that the temperature is held constant?
(A) Bromomethane
(B) Bromoethane
(C) 1-Bromopropane
(D) They all condense at the same pressure.
28. Which attractive force is least likely to be the cause of condensation?
(A) Hydrogen bonding
(B) London forces
(C) Dipole-dipole attractive forces
(D) Dipole-induced dipole attractive forces
Questions 29–30 refer to the diagrams below.
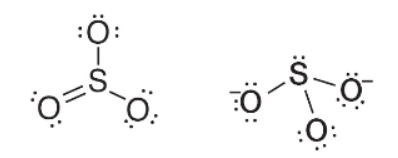
29. The Lewis electron-dot structures, ignoring formal charges, for sulfur trioxide and for the sulfite ion are given above. Which statement best describes the geometry of these two substances?
(A) The sulfite ion has a triangular pyramid shape, and the sulfur trioxide is a planar triangle.
(B) Both substances are flat, trigonal, planar shapes.
(C) Both substances are triangular pyramids.
(D) SO3 is a triangular pyramid, and 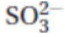 is a planar triangle.
is a planar triangle.
30. Which statement below describes the charge, polarity, and resonance characteristics of the sulfite ion and of the sulfur trioxide species shown above?
(A) The sulfite ion has two negative charges along with a shape that makes it a dipole. Sulfur trioxide is symmetrical and nonpolar. In addition, as written, sulfur trioxide has three resonance structures.
(B) The sulfite ion and sulfur trioxide are both polar with the only difference being that one is an ion and the other is not.
(C) Sulfur trioxide has three resonance structures, and the sulfite ion has no resonance structures.
(D) Sulfite ions have a nonbonding electron pair domain, while sulfur trioxide has all electron domains as bonding domains.
Questions 31–33 refer to the following equation.
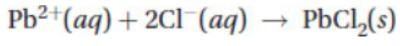
A chemist mixes a dilute solution of lead(II) perchlorate with an excess of potassium chloride to precipitate lead(II) chloride (Ksp = 1.7 × 10-5). The net ionic equation is given above for the reaction.
31. Which of the following is a molecular equation for this reaction?
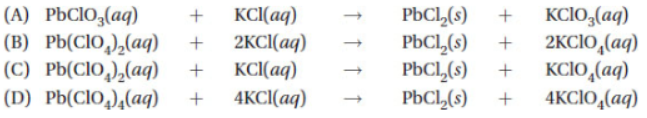
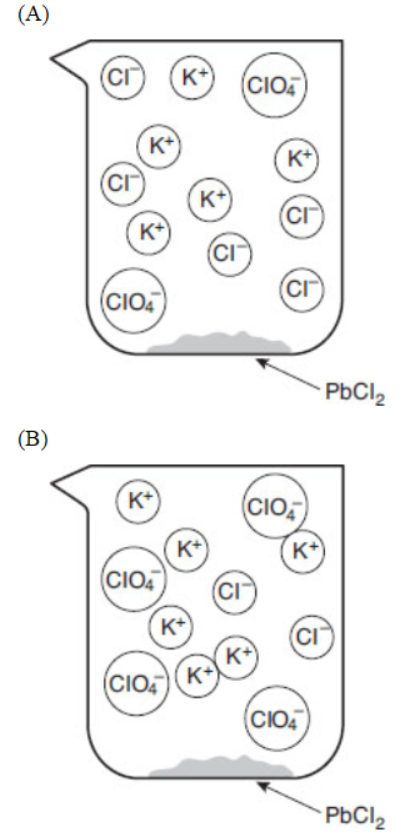
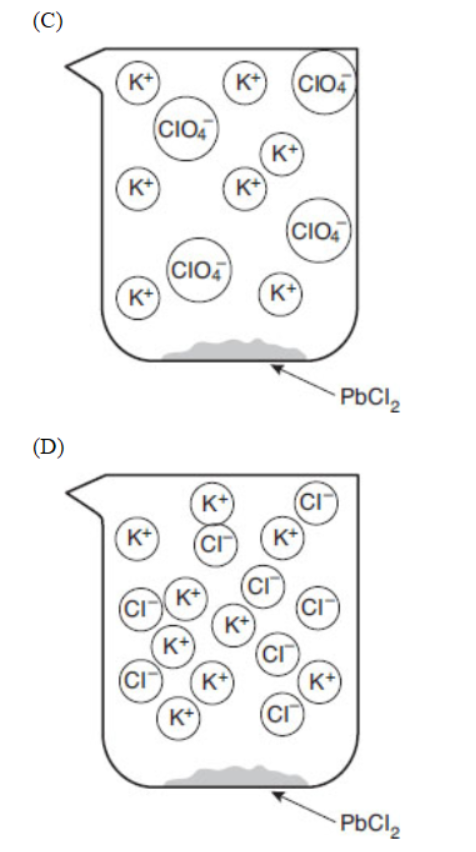
32. Which of the following particulate views represents the experiment after the reactants are mixed thoroughly and the solids are given time to precipitate?
33. Within a factor of ten, what is the approximate molar solubility of lead(II) chloride in distilled water?
(A) 10-2 mol/L
(B) 1.7 × 10-5 mol/L
(C) 10-7 mol/L
(D) 10-15 mol/L
Questions 34–38 refer to the following equation.
N2O4(g) ⇌ 2NO2(g)
N2O4(g) decomposes into NO2(g) according to the equation above. A pure sample of N2O4(g) is placed into a rigid, evacuated, 0.500 L container. The initial pressure of the N2O4(g) is 760 torr. The temperature is held constant until the N2O4(g) reaches equilibrium with its decomposition products. The figure below shows how the pressure of the system changes while reaching equilibrium.
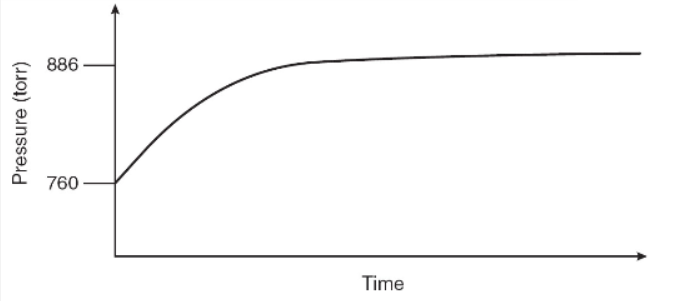
34. Why does the pressure rise in this experiment?
(A) The intermolecular attractions inside the container decrease, so the molecules strike the walls more frequently.
(B) The intermolecular attractions inside the container increase, which increases the force as molecules collide with the walls of the container.
(C) The average kinetic energy increases as the reaction continues.
(D) The number of particles striking the container walls per unit time increases.
35. The figure above gives us information about all the following EXCEPT
(A) the activation energy
(B) the reaction rate
(C) the position of equilibrium
(D) the order of reaction
36. By how much will the equilibrium pressure increase if this reaction goes to completion?
(A) 634 torr
(B) 760 torr
(C) 836 torr
(D) 900 torr
37. What can be said about the equilibrium constant, Kp, for this reaction?
(A) Kp > 1
(B) Kp < 1
(C) Kp = 1
(D) The data do not allow us to estimate the value of Kp.
38. Which diagram is an appropriate description of the system if more NO2(g) is rapidly injected, at time = 0, into the container after equilibrium is established?
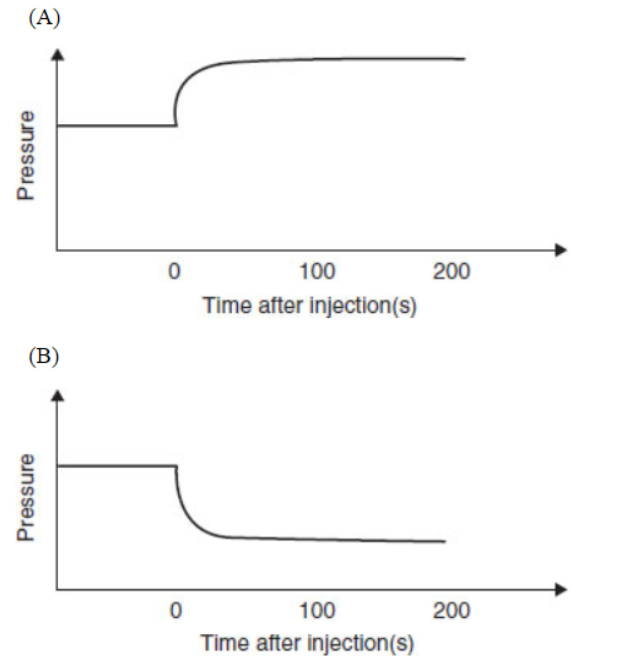
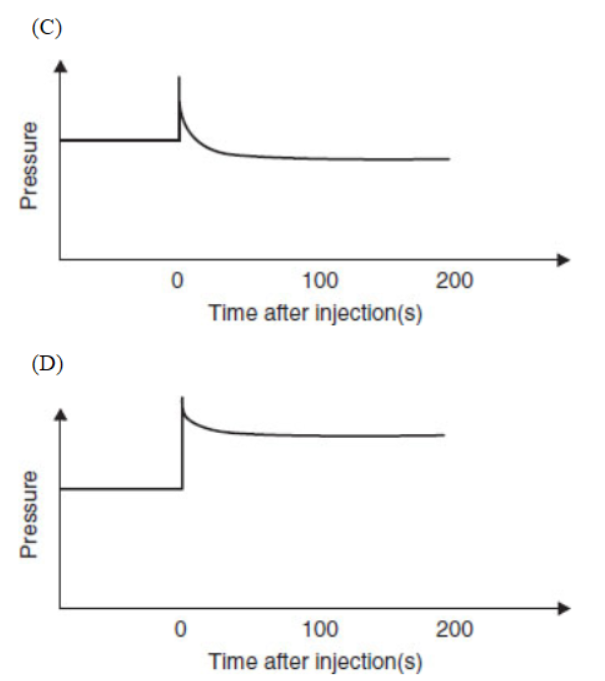
Questions 39–43 refer to the following information.
This formation equation for the reaction synthesizing RbBr(s) can be separated into a series of steps.
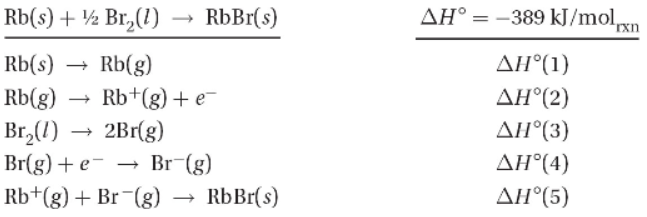
39. Which of the steps in the table above are endothermic?
(A) ∆H°(1) and ∆H°(2) only
(B) ∆H°(1), ∆H°(2), and ∆H°(3) only
(C) ∆H°(3) only
(D) ∆H°(3) and ∆H°(4) only
40. If this reaction goes to completion, producing RbBr from the reactants, and if you use the overall chemical equation to estimate the entropy change for this process, which of the following statements is correct?
(A) The reaction is favorable and driven by the enthalpy change since the entropy decreases in this process.
(B) The reaction is unfavorable since the entropy change is a large negative value.
(C) The reaction is favorable and driven by both enthalpy and entropy changes.
(D) The reaction is unfavorable because of the enthalpy and entropy changes.
41. Using Hess’s law, which of the following combinations will give the enthalpy for the following reaction?
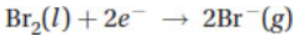
(A)∆H° = ∆H°(3) + ∆H°(4)
(B)∆H° = 2∆H°(3) + ∆H°(4)
(C)∆H° = ∆H°(3)/2 + ∆H°(4)
(D)∆H° = ∆H°(3) + 2∆H°(4)
42. When 0.100 mol of Br2(l) is formed from RbBr in the reaction above, how much heat is released or absorbed?
(A) 77.8 kJ of heat is released.
(B) 77.8 kJ of heat is absorbed.
(C) 38.9 kJ of heat is released.
(D) 38.9 kJ of heat is absorbed.
43. If 1.0 g of rubidium and 1.0 g of bromine are reacted, what will be left in measurable amounts (more than 0.10 mg) in the reaction vessel?
(A) RbBr only
(B) RbBr and Rb only
(C) RbBr and Br2 only
(D) RbBr, Rb, and Br2
Question 44 refers to the following information.
Formation of a solution can often be visualized as a three-step process.
Step 1. Solvent molecules are separated from each other to make space for the solute.
Step 2. Solute molecules are separated so they fit into spaces in the solvent.
Step 3. Separated solute and solvent are brought together, filling in the spaces.
44. Which of the following statements about the energy involved in the above is correct?
(A) Steps 1 and 2 are exothermic, and step 3 is endothermic.
(B) Steps 1 and 2 are endothermic, and step 3 is exothermic.
(C) All three steps are exothermic.
(D) All three steps are endothermic.
______________________________________________________________________
45. Nickel (Z = 28, A = 59) has a first ionization energy of 737 kJ/mol and a boiling point of 2913 °C, while Pt (Z = 78, A = 195) has a first ionization energy of 870 kJ/mol and a boiling point of 3825 °C. Which of the following are the most reasonable values of ionization energy and boiling point for palladium?
(A) -200 kJ/mol and 3524 °C
(B) 795 kJ/mol and 2436 °C
(C) 804 kJ/mol and 2963 °C
(D) 1284 kJ/mol and 3416 °C
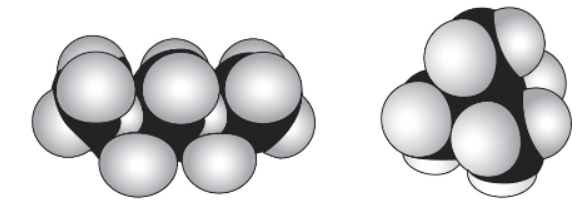
46. n-pentane (C5H12 or CH3CH2CH2CH2CH3) and 2,2-dimethylpropane (C5H12 or (CH3)4C), shown above as space-filling models, each have the same number of carbon and hydrogen atoms but the atoms are arranged differently. n-pentane boils at 36.1 °C, and 2,2-dimethylpropane boils at 9.5 °C. Which statement best explains these data?
(A) The long chains of n-pentane make it more difficult for them to reach the liquid surface and vaporize.
(B) The long chains of n-pentane provide more sites for London attractive forces for neighboring molecules to have an effect on each other.
(C) The compact structure of the 2,2-dimethylpropane directs the attractive forces internally in the molecule.
(D) The bonds in the n-pentane are weaker, allowing small parts of the molecule to vaporize easily.
Use the following for questions 47–49.
A galvanic (voltaic) cell is described by the cell diagram below.
A molecular-level view of the silver electrode is given.
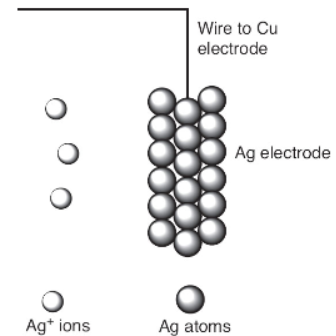
47. Which of the following best represents what happens to the silver metal electrode when the copper and silver electrodes are attached by a wire?
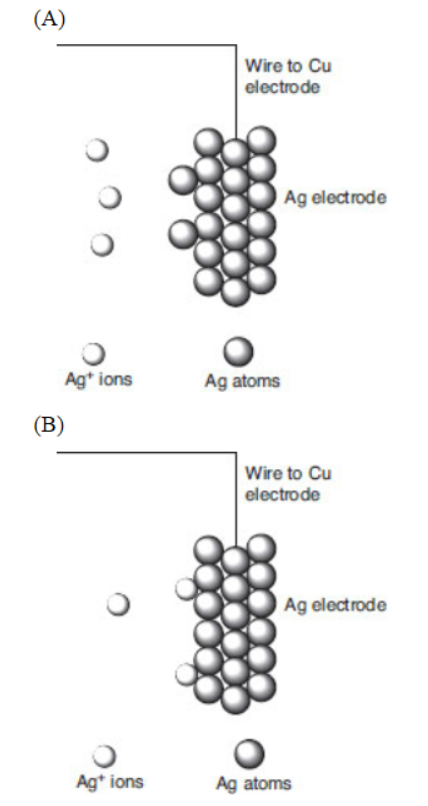
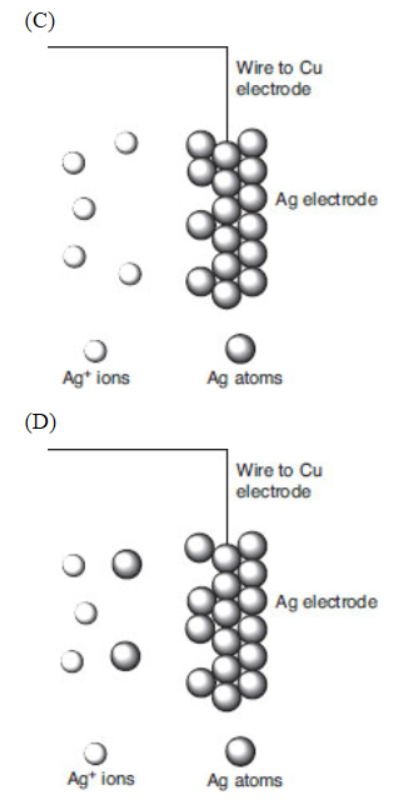
48. Which particulate view indicates that silver ions are being reduced?
.png)
.png)
49. Which particulate view indicates that silver atoms are being oxidized?
.png)
.png)
50. What is the purpose of a salt bridge in a galvanic cell?
(A) To separate the two cells
(B) To equalize the charge between the two cells as the redox process occurs
(C) To allow the reducing substance to flow to the other cell to react with the oxidizing material
(D) To allow electrons to flow from one cell to another to complete the circuit
Magnesium has an atomic radius of 160 pm and a first ionization energy of 737 kJ.
51. Based on periodic trends and the data given above, what are the most probable values for the ionization energy and atomic radius of sodium?
(A) 186 pm, 496 kJ/mol
(B) 186 pm, 898 kJ/mol
(C) 135 pm, 523 kJ/mol
(D) 147 pm, 523 kJ/mol
Iron is slowly oxidized by the permanganate in the reaction
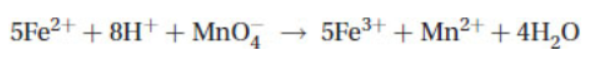
The decrease in absorbance of a dilute solution of permanganate is followed using a spectrometer set to the appropriate wavelength in the visible region of the spectrum. The table below provides the data collected in this experiment.
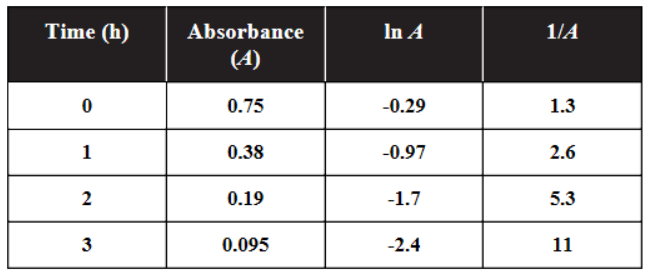
52. Which of the following is the best interpretation of the data?
(A) The data support a first-order reaction.
(B) The data support a second-order reaction.
(C) The data support a zero-order reaction.
(D) The overall order is 14.
53. 0.0025 mol of a weak, monoprotic acid is dissolved in 0.250 L of distilled water. The pH was then measured as 4.26. What is the pKa of this weak acid?
(A) 4.26
(B) 6.52
(C) 7.92
(D) 8.52
Questions 54–57 refer to the data and graph below.
Four different acid solutions of 0.0100 M are prepared, and their pH values are recorded on a laptop computer. One of the solutions contains more than just an acid. At the point designated as “add,” all four solutions are diluted with an equal volume of water. The bottom line represents solution 1, and the top line represents solution 4.
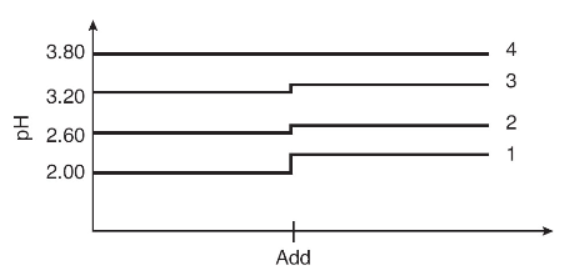
54. Which of the four acids is best described as a strong acid?
(A) Solution 1 since the initial pH is 2.00
(B) Solution 2 since there is a small pH change
(C) Solution 3 since the initial pH is highest and changes when water is added
(D) Solution 4 since added water doesn’t affect the pH
55. Using the data in the graph, which solution seems to be a buffer?
(A) Solution 1 since the solution is the most dilute
(B) Solution 2 since water is a base and increases pH
(C) Solution 3 since the pH is highest with a pH change
(D) Solution 4 since added water has no effect on buffers
56. Which solution contains only an acid and the acid is the weakest of all?
(A) Solution 1 since the weakest acid will have the largest pH change
(B) Solution 2 since water changes the pH the least and therefore is the weakest
(C) Solution 3 since the initial pH is the largest and the added water changes the pH
(D) Solution 4 since this acid is so weak that water has no effect on the pH
57. Which acid(s) will require the most 0.0050 M NaOH to neutralize 25.0 mL of a 0.010 M solution of the acid?
(A) Solution 1
(B) Solution 2
(C) Solution 3
(D) They will all require the same volume of NaOH.
______________________________________________________
58. Sulfurous acid is a weak acid, while sulfuric acid is a much stronger acid because
(A) the sulfur in sulfuric acid is more electronegative than the sulfur in sulfurous acid
(B) sulfuric acid has more oxygen atoms in its formula
(C) the O–H bonds in sulfuric acid are much weaker than those in sulfurous acid
(D) sulfurous acid has its hydrogen atoms bound directly to the sulfur atom
59. You cannot prepare a buffer by
(A) mixing a solution of a weak base with a strong acid
(B) mixing a solution of a weak acid with a strong base
(C) mixing a solution of a strong base with a strong acid
(D) mixing a solution of a weak acid with its conjugate base
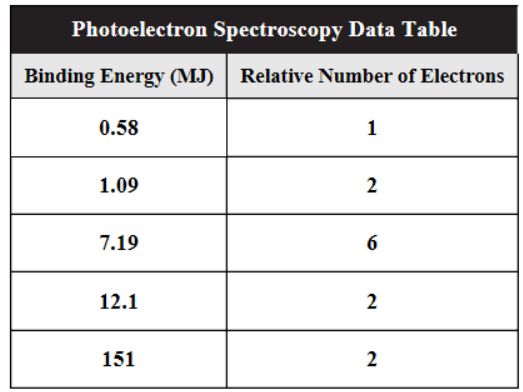
60. The photoelectron data table for aluminum is shown above. Which of the following statements is not true about these data?
(A) All 13 electrons are listed.
(B) The three valence electrons have the lowest energies at 0.58 MJ/mol and at 1.09 MJ/mol.
(C) The p electrons are all at the same energy of 7.19 MJ/mol.
(D) It takes 0.58 MJ to remove a valence electron.

Section II: Free-Response
Start only when you have 105 minutes to complete the whole section.

Instructions
The questions appear on the following pages. You may use the periodic table and the table of equations and constants that were provided with Section I. On the actual exam, you will be directed to write your final answers only in the test booklet. For this practice test, write your answers on separate sheets of lined paper. You will need about three pages for each of questions 1 through 3 and one page for each of questions 4 through 7.
Do not spend too much time on any one question. Budget your time carefully, and answer the easier questions first.
Be sure that your work is well organized, complete, and easy to read. Cross out or erase any mistakes. Erased and crossed-out material will not be scored.
Section II
7 Free-Response Questions
(Time: 105 minutes)
CALCULATORS ARE ALLOWED FOR SECTION II

1. Electrochemistry is used in a variety of ways to represent how substances react. Below is an oxidation-reduction (redox) chemical equation and an unlabeled diagram of a galvanic (voltaic) cell with aluminum and magnesium electrodes. The standard reduction potential of Al3+ + 3e- → Al° is -1.66 V, and the standard reduction potential for Mg2+ + 2e- → Mg° is -2.37 V.
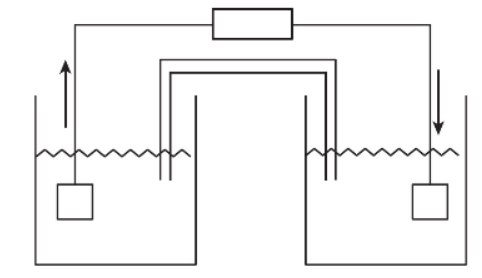
Early studies of galvanic cells were done with voltmeters that could report only positive voltages. Therefore, it was common to present a galvanic cell with a positive voltage.
(a) Calculate the standard cell voltage, 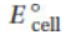 , for these substances, and enter it on the meter in the diagram.
, for these substances, and enter it on the meter in the diagram.
.png)
(b) Write the balanced net ionic equation.
(c) Identify the cathode and the metal it is made of.
(d) Will the cathode increase or decrease in mass if electrons flow? Justify your conclusion.
(e) Identify the salt bridge, and describe its purpose. Place + and - charge signs on the appropriate electrodes.
(f) If the voltmeter is short circuited with a wire so that the reaction will occur, what will the voltage reading be when equilibrium is reached?
(g) Calculate the value of ΔG° for this reaction.
2. The balanced equation for the reaction of potassium permanganate with oxalate acid occurs in a solution made acidic with sulfuric acid.
(a) Write the ionic equation.
(b) Write the net ionic equation.
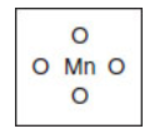
(c) Give the Lewis electron-dot structure of the 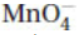 ion shown in the following box. (Do not forget the charge.)
ion shown in the following box. (Do not forget the charge.)
(d) What mass of KMnO4 must be weighed to produce 250. mL of 0.0080 M KMnO4? Show your calculations. (The molecular mass of KMnO4 is 158.04 g/mol.)
(e) If 0.008 M solutions of H2C2O4 and H2SO4 are also available for this experiment and if equal volumes of the three reactants are used, which is the limiting reactant? Show your reasoning by using a calculation.
(f) An experiment is performed to determine the enthalpy of the oxidation-reduction reaction above by measuring 25.0 mL of each reactant into separate beakers. When all three beakers equilibrate at room temperature, an electronic temperature-measuring device is placed into the H2SO4 beaker, and then the other two beakers are rapidly mixed with the beaker that has the temperature sensor. After the three solutions are mixed, the temperature of the reaction is recorded, as shown in the figure below. Answer questions about the temperature curve.
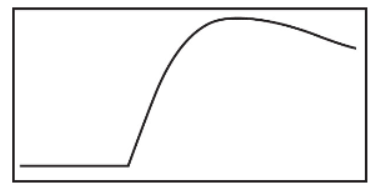
(i) What does this horizontal line represent?
(ii) What factors contribute to the curved temperature line?
(g) What last piece of information is needed to determine the enthalpy of the reaction,  ? Explain your answer.
? Explain your answer.
3. The partial photoelectron spectrum is represented below.
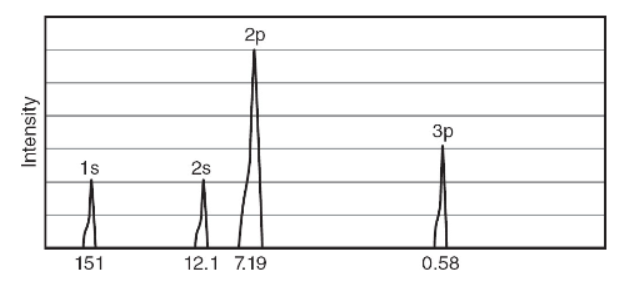
(a) Add a sketch of the missing peak at its correct position on the spectrum above, and justify your decision.
(b) Write out the ground state electron configuration for this element.
(c) Identify the element that this electron configuration represents.
(d) Calculate the wavelength of an electron ejected from the 2p orbital for this element. Show your work, and include the correct units.
(e) Can the first ionization energy (IE) of this element be determined? Explain why and how.
(f) Why do all the 2p electrons represented by a single peak in a PES spectrum of an element have the same energy?
(g) Why do the 2p electrons (in every element after Be) have different PES wavelengths?
4. The graph below lists the boiling point of the hydrides of the elements in groups 4-7.
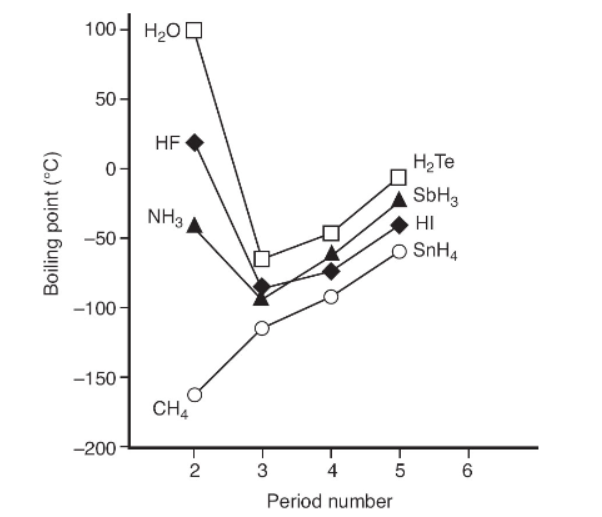
(a) Why is the boiling point a better indicator than the melting point, density, polarity, or color in judging intermolecular attraction forces? Be sure to consider all factors.
(b) In the graph above, which substances have unexpectedly high intermolecular attractive forces? How did you come to your conclusions?
(c) Why does water have the highest intermolecular attractive forces? Consider the information from the graph. How it is also explicable based on molecular structure?
(d) How many hydrogen bonds can water participate in with other molecules? How many hydrogen bonds can HF and NH3 participate in with other molecules? Illustrate your answer with diagrams showing intermolecular bonds with dashed lines and covalent bonds with solid lines.
5. Simple molecules with a single central atom have well-known structures (shapes) that are often described by their electron domains (both bonding and nonbonding). In the given boxes, draw the Lewis electron-dot structures for the following substances (show bonding and nonbonding electrons). Be sure to show the correct shape, write the name of the shape, and give the name of the molecule.
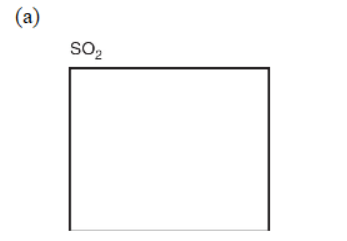
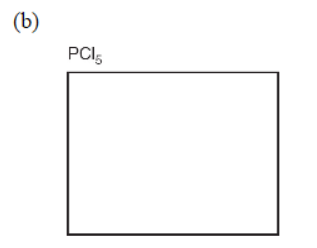
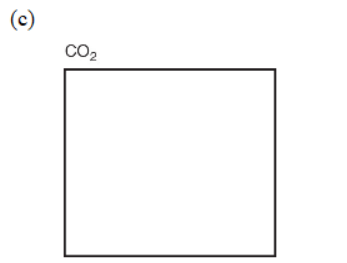
(e) Some substances can be represented as several equivalent arrangements of bonds, called resonance structures. Sulfur trioxide (SO3) can be depicted as a group of resonance structures. Draw all the possible Lewis electron-dot resonance structures for SO3 along with the resonance arrows between them.
6. Ammonia, NH3(g), is a weak base when dissolved in water.
(a) Write the equation for the dissolution of NH3(g) to form the aqueous solution NH3(aq). Next, write the equation for the base NH3(aq) reacting with water to form its conjugate acid.
(b) Write the Kb expression for an ammonia solution. (The value of Kb is 1.8 × 10-5.)
(c) Calculate the concentration of hydroxide ions of a 0.30 M ammonia solution. Show your work, indicate all assumptions made, and justify that all assumptions were reasonable.
(d) What is the value of the Ka of the conjugate acid of NH3? Also, what is the value of the pKa of the conjugate acid of NH3?
7. NO(g) reacts with H2 to form N2 + 2H2O in the following reaction.
2NO(g) + 2H2(g) ® N2(g) + 2H2O(g)
Several experiments yielded the results shown in the table below.
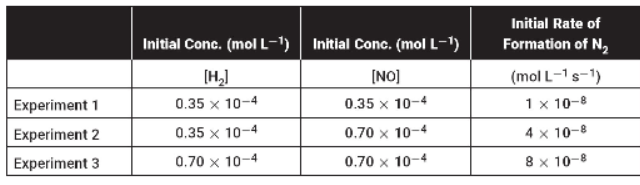
(a) Why are initial reaction rates measured in this experiment?
(b) What is the rate law for this reaction?
(c) What is the overall order of this reaction, and what are the order of the H2 and the order of the NO?




