Practice Exercises - Structure of the Atom - AP Chemistry Premium 2024
Multiple-Choice
1. Albert Einstein was given the Nobel Prize for the discovery and explanation of the photoelectric effect. What is this effect?
(A) It is spectroscopy using visible light.
(B) It is the ejection of electrons from a surface by photons.
(C) It measures E = mc2 for any element.
(D) It is another way of stating the Heisenberg uncertainty principle.
2. Which orbital diagram represents the outer electrons of the ground state of an element?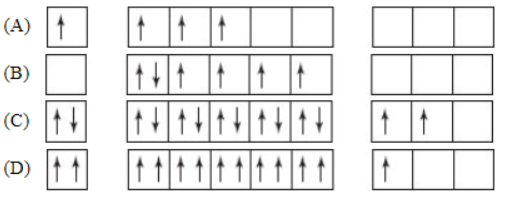
3. The wave-particle duality of nature applies to only
(A) everything
(B) light because of its wave properties
(C) gamma rays since they are the most energetic electromagnetic radiation
(D) electrons because they determine the chemical nature of the elements
4. Which of the following best depicts a p orbital?
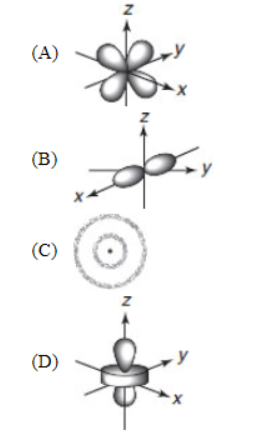
5. Calcium can have an electron in an f orbital
(A) if the calcium is in the elemental state
(B) if the calcium is in an excited state
(C) if the calcium is a positive ion
(D) if the calcium is a negative ion
6. What is the complete electron configuration for silicon?
(A) [Ne] 3s2 3p2
(B) 1s2 2s2 2p6 3s2 3p4
(C) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
(D) 1s2 2s2 2p6 3s2 3p2
7. Which electromagnetic wave has the highest energy? The x-axis is time, and the y-axis is amplitude.
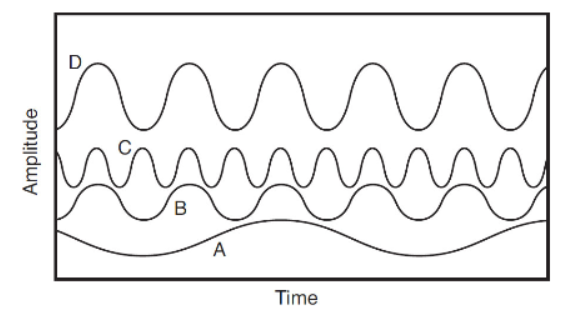
(A) Wave A
(B) Wave B
(C) Wave C
(D) Wave D
8. What is the wavelength of light that has a frequency of 4.00 × 1014 s−1? (The speed of light is 3.00 × 108 m s−1.)
(A) 7.5 nm because this is the result of speed divided by frequency
(B) 1,333 nm because this is the result of frequency divided by speed
(C) 750. nm when the correct metric prefix is used
(D) 1.33 cm−1 since the speed of light should have units of cm/s
9. Which of the following elements has the greatest number of p electrons?
(A) Arsenic since it has the highest atomic number and so the others can’t have more
(B) Silicon since it is alphabetically last in this group
(C) Iron since it has the most d electrons
(D) Chlorine since it has 5 p electrons in its period
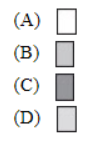
10. Which of the following shows the valence electrons of an uncharged atom of Sb?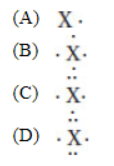
11. Which region of the expanded periodic table has elements that have d electrons listed last in their electron configurations?
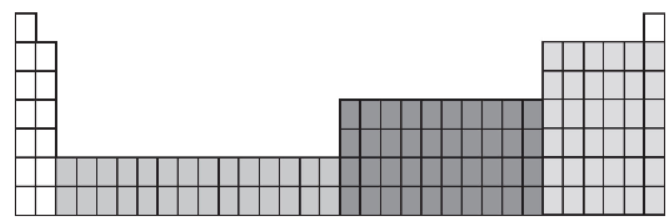
12. How many protons, neutrons, and electrons does the neutral atom of the chromium-52 isotope have (also written as 52Cr)?
(A) 52 p, 28 n, and 52 e−
(B) 28 p, 24 n, and 24 e−
(C) 26 p, 26 n, and 26 e−
(D) 24 p, 28 n, and 24 e−
13. Which element has an electronic configuration that has the largest number of unpaired electrons?
(A) Fe because it is in the middle of the d block and has 4 unpaired electrons
(B) Al since it forms the Al3+ ion and has all unpaired electrons
(C) Ag because silver has 9 d electrons and 9 unpaired electrons
(D) Ni since it has 2 unpaired electrons
14. Which electronic configuration corresponds to that of a noble gas?
(A) 1s2 2s2 2p6 3s2 3p6 4s1 because it has the most energy levels
(B) 1s2 2s2 2p6 3s2 3p4 since it ends with s and p electrons
(C) 1s2 2s2 2p6 3s2 3p6 since it has the ns2np6 structure of a noble gas
(D) 1s2 2s2 2p6 3s1 since it has the characteristic ns np structure of a noble gas
15. Which electronic transition requires the addition of the most energy?
(A) n = 1 to n = 3 because this has the largest energy increase
(B) n = 5 to n = 2 since this has the largest size decrease
(C) n = 2 to n = 3 because this has the smallest mass increase
(D) n = 4 to n = 1 because this has the largest momentum decrease
16. What is the maximum number of valence electrons that any atom may possess?
(A) 32 is the maximum number of electrons because the s orbital can have 2 electrons, the p orbital can have 6 electrons, the d orbital can have 10 electrons, and the f orbital can have 14 electrons, which all add up to 32 electrons.
(B) 2 valence electrons are the most that an s orbital can contain.
(C) 14 f electrons are in the largest orbital.
(D) 2 s electrons and 6 p electrons add up to 8, which is the maximum number of valence electrons possible.
17. Which was used to determine the charge of the electron?
(A) The gold foil experiment
(B) Deflection of cathode rays by electric and magnetic fields
(C) The oil drop experiment
(D) The mass spectrometer
18. Which of the following principles is NOT part of Dalton’s atomic theory?
(A) Chemical reactions are simple rearrangements of atoms.
(B) Atoms follow the law of multiple proportions.
(C) Each atom of an element is identical to every other atom of that element.
(D) All matter is composed of atoms.
19. Which statement is correct?
(A) Technetium, Tc, has 15 p electrons.
(B) Argon has 18 valence electrons.
(C) Oxygen has 4 electrons.
(D) Tungsten has 14 f electrons.
20. Which quantum number describes the shape of an orbital?
(A) The principal quantum number, n
(B) ℓ, which is the second term in an electron configuration
(C) mℓ, because it tells the number of directions to which the suborbitals point
(D) ms, which is the spin quantum number
21. You have just discovered a new, fundamental particle of nature. When measuring its mass, you obtain the following data for four samples:
4.72 × 10−34 gram
9.44 × 10−34 gram
1.18 × 10−33 gram
1.65 × 10−33 gram
Millikan found the mass of an electron by finding the common factor in a mass of data. What is the common factor in the data presented?
(A) 1.18 × 10−34 g
(B) 2.36 × 10−34 g
(C) 4.72 × 10−34 g
(D) 9.44 × 10−34 g

22. Which of the following is FALSE?
(A) The 4d orbitals are in the fourth period of the periodic table.
(B) The 7s orbitals are in the seventh period of the periodic table.
(C) The 4f orbitals are in the sixth period of the periodic table.
(D) The 6s orbitals are spherical in shape.
23. The f sublevel may contain a maximum of
(A) 14 electrons
(B) 10 electrons
(C) 6 electrons
(D) 2 electrons
24. The valence electrons are
(A) all electrons in an atom beyond the preceding noble gas
(B) all outermost electrons in a sublevel
(C) s and any p electrons in the highest energy level or shell
(D) electrons in the last unfilled sublevel
25. Which equation best expresses the energy of a photon?
(A) 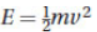
(B) E = mc2
(C) E = IR
(D) E = hn
Free-Response
Answer the following question concerning the structure of the atom, the use of light as a probe of atomic structure, and the development of the modern quantum mechanical view of the atom.
(a) Describe the significant experiments that led to the discovery of the properties of the electron, proton, and neutron. Describe the results and the interpretation of results that contributed to development of the atomic model.
(b) The bond energy of a carbon-carbon bond is approximately 350 kJ/mol. Explain why electromagnetic radiation with wavelengths shorter than those in the visible region of the spectrum is often called ionizing radiation.
(c) Sketch an outline of the periodic table, and discuss how the four quantum numbers n, ℓ, mℓ, and ms relate to the periodic table.
(d) If an electron drops from the fifth energy level to the second energy level in a hydrogen atom, will energy be released or absorbed? Will the energy of the electron increase or decrease? Explain your answers.




