Practice Exercises - Solutions - AP Chemistry Premium 2024
Multiple-Choice
1. The solubility of cadmium chloride, CdCl2, is 140 g per 100 mL of solution. What is the molar solubility (molarity) of a saturated solution of CdCl2?
(A) 0.765 M
(B) 1.31 M
(C) 7.64 M
(D) 12.61 M
2. Which of the following represents the most dilute solution if  represents the solvent and
represents the solvent and  represents the solute?
represents the solute?
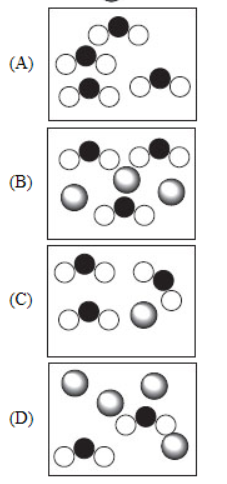
3. To make a solution, 3.45 mol of C6H13Cl and 1.26 mol of C5H12 are mixed. Which of the following is needed, but not readily available, to calculate the molarity of this solution?
(A) The density of the solution
(B) The densities of C6H13Cl and C5H12
(C) The temperature
(D) The molar masses of C6H13Cl and C5H12
4. If a solute dissolves in an endothermic process
(A) hydrogen bonds must exist between solvent and solute
(B) the entropy of the solution must be greater than that of its pure components
(C) the entropy of the solution is not a factor
(D) strong ion-dipole forces must exist in the solution
5. Which of the following concentration values changes as the temperature of a solution changes?
(A) Mole fraction
(B) Molarity
(C) Molality
(D) Mass percent
6. When algae decay in a pond, the process uses up the available oxygen. Which of the following factors will also contribute to a decrease in oxygen in a pond?
(A) Decreasing salinity (salt concentration)
(B) Increasing acidity due to acid rain
(C) Increasing temperature
(D) Increasing surface tension of the water
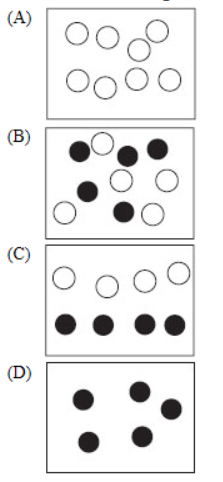
7. Which of the following is the best representation of a solution?
8. What is the correct process to make a 0.500 L solution of 1.0 M glucose? (Molar mass = 180.1 g mol−1)
(A) Dissolve 180.1 g of glucose in 1.00 kg of distilled water.
(B) Dissolve 90.0 g of glucose in enough distilled water to produce 0.500 L of solution.
(C) Dissolve 90.0 g glucose in 0.500 kg of distilled water.
(D) Dissolve 90.0 g of glucose in 0.500 L of distilled water.
.png)
9. When KCl dissolves in water, the solution cools noticeably to the touch. It may be concluded that
(A) the entropy increase overcomes the unfavorable heat of dissolution
(B) KCl is relatively insoluble in water
(C) the entropy decreases when KCl dissolves
(D) the boiling point of the solution will be less than 100 °C
Free-Response
Use the fundamental concepts of this chapter to consider solutions and their behaviors.
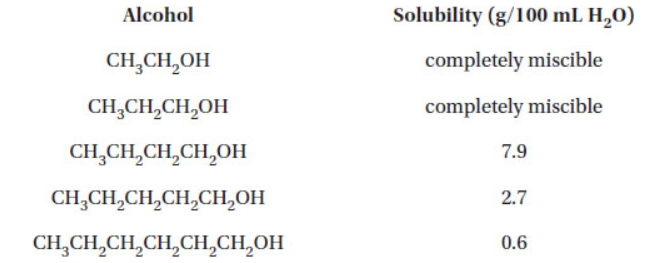
(a) Given the data below, explain why the solubility of alcohols decreases as the length of the carbon chain increases.
(b) A student is asked to make an aqueous solution of potassium chloride by dissolving 5.8 g of KCl in enough water to make 250. mL of solution.
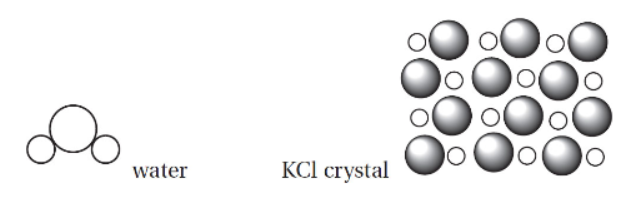
(i) Draw a diagram of the different particles existing in the solution. Base your illustration on the figures above. Use only a single formula unit and a maximum of 8 water molecules. Label all the ions and the proper orientation of the solvent.
(ii) What is the molar concentration of the aqueous potassium chloride solution?




