Practice Exercises - Ionic Compounds, Formulas, and Reactions - AP Chemistry Premium 2024
Multiple-Choice
1. What is the correct formula for aluminum sulfate?
(A) Al3(SO4)2 where the subscripts were chosen to match the ion charges
(B) AlSO4 since ionic compounds are in 1 to 1 ratios of the ions
(C) Al2(SO4)3 where the subscripts were chosen so the positive charge and negative charge add to zero
(D) Al2(SO3)3 where the subscripts were chosen so the positive charge and negative charge add to zero
2. What are the spectator ions that do not participate in the net ionic equation when solutions of potassium dichromate (K2Cr2O7) and lead nitrate (Pb(NO3)2) are mixed?
(A) Pb2+ and K+
(B) 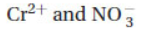
(C) Cr2+ and K+
(D) 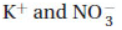
3. Which of the following will act as a driving force for a chemical reaction?
(A) Formation of a gas that bubbles out of solution
(B) Formation of a weak electrolyte that removes two ions from solution
(C) Formation of water, which is a weak electrolyte
(D) All of these are driving forces
4. Which compound, made from the indicated pairs of ions, is expected to be the least soluble based on coulombic attractive forces?
(A) Iron(III) and nitride ion because both ions have 3 charges of opposite sign
(B) Bromide ion and sodium ion because both ions have single charges of opposite sign
(C) Calcium ion and bromide ion because one ion with a +2 charge is attracting 2 ions each with a -1 charge
(D) Bromide ion and iron(III) because one ion with a +3 charge is attracting three ions each with a -1 charge
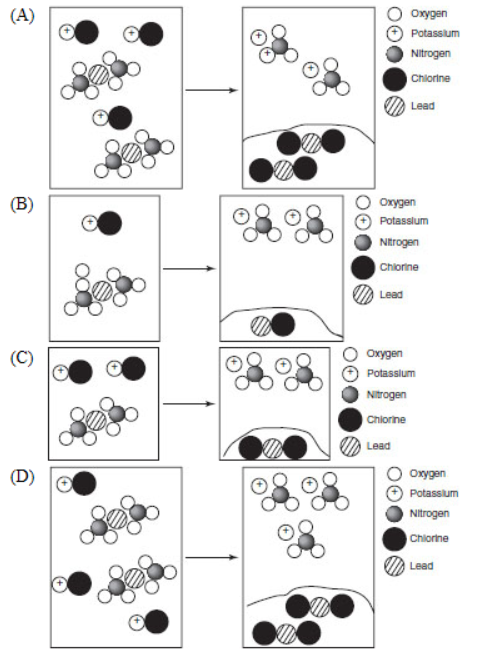
5. When lead(II) nitrate is mixed with potassium chloride, which of the following representations illustrates the correct ratios and solubility of the products?
6. When ammonium carbonate, (NH4)2CO3, is dissolved in water, the ions formed are
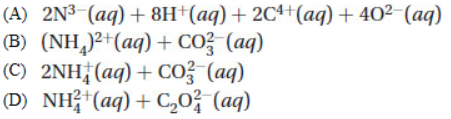
7. Which of the following ions are isoelectronic to (have the same electron configuration as) neon?
(A) F−
(B) Ga3+
(C) P3−
(D) All of these are isoelectronic to neon.
8. Which substance is expected to have the largest distance between its nuclei?
(A) NaF
(B) NaCl
(C) NaI
(D) NaBr
9. Which of the following pairs of compounds is expected to have the compound with largest solubility listed first?
(A) MgCl2 and AlBr3
(B) CaF2 and MgS
(C) KBr and Na2S
(D) Al2O3 and Cr2O3
10. Which two atoms form ions with the same 1s2 2s2 2p6 electron configuration?
(A) Cl and Na
(B) Cl and F
(C) Na and F
(D) S and Br
11. What is the formula and name for an ionic compound formed from aluminum and chlorine?
(A) AlCl, aluminum chloride
(B) Al3Cl, tri-aluminum chloride
(C) Al3Cl3, aluminum(III) trichloride
(D) AlCl3, aluminum chloride
12.The electronic configuration 1s2 2s2 2p6 3s2 3p6 corresponds to the electronic configuration of
(A) O2-
(B) F−
(C) Na+
(D) all of the above
13. Which of the following reactions is a decomposition reaction?
(A) 2KClO3(s) → 2KCl(s) + 3O2(g)
(B) C7H8O2(l) + 8O2(g) → 7CO2(g) + 4H2O(l)
(C) 2Cr(s) + 3Cl2(g) → 2CrCl3(s)
(D) 6Li(s) + N2(g) → 2Li3N(s)
14. Which of the following is NOT a correct chemical formula and why?
(A) SrBr2; strontium has only ions with a +1 charge
(B) Ca2O3; the charges for the ions do not add up to zero
(C) Mg3N2; the charges for the ions do not add up to zero
(D) Na2S; sodium cannot bond with sulfur
15. Which of the following is a correct formula?
(A) KH2PO4
(B) CaC2H3O2
(C) Na2ClO4
(D) Ba(CO3)2
16. Which of the following molecular representations show pairs of substances undergoing a reaction often classified as single replacement?
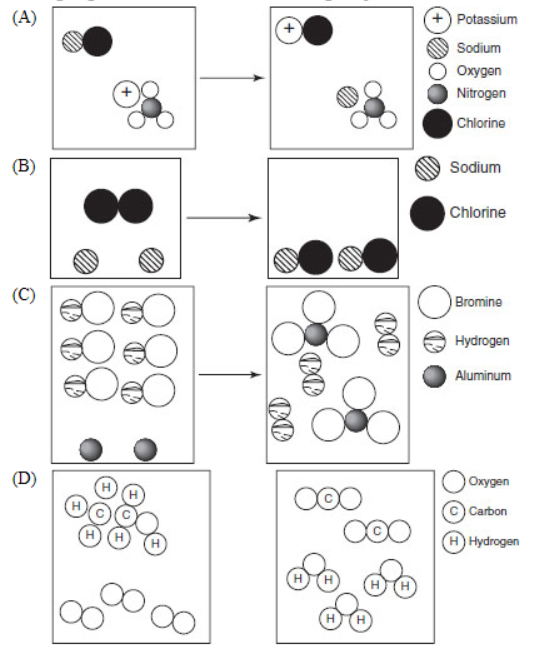
17. Which of the following is NOT true of a net ionic equation?
(A) All of the nonreacting (spectator) ions have been canceled.
(B) It shows the actual reactants in an equation.
(C) It allows the chemist to substitute reactants in a logical manner.
(D) It is used to determine which compounds are insoluble.
.png)
18. What is the balanced molecular equation when FeCl3(aq) is mixed with Ba(OH)2(aq)?
(A) Ba(OH)2(aq) + FeCl3(aq) → Fe(OH)2(aq) + BaCl3(aq)
(B) 2Ba(OH)2(aq) + 3FeCl3(aq) → 2BaCl2(aq) + 3Fe(OH)3(s)
(C) 3Ba(OH)2(aq) + 2FeCl3(aq) → 3BaCl2(s) + 2Fe(OH)3(s)
(D) 3Ba(OH)2(aq) + 2FeCl3(aq) → 3BaCl2(aq) + 2Fe(OH)3(s)
19. Gaseous hydrogen and nitrogen gas are reacted to form ammonia. Which of the following molecular views shows the correct stoichiometry for this reaction?
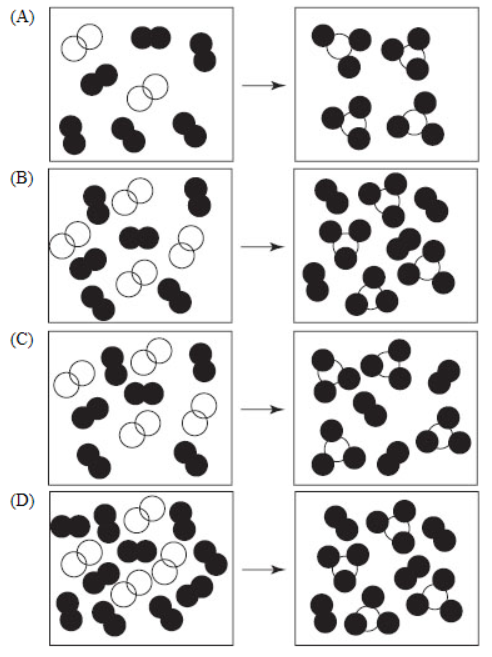
20. Which of the following molecular views does not violate the law of conservation of matter?

21. What is the net ionic equation for the following reaction that takes place in aqueous solution?
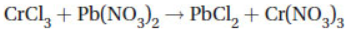
(A) Cr2+(aq) + Pb2+(aq) → CrPb(s)
(B) Cr3(aq) + 3NO3(aq) → Cr(NO3)3(s)
(C) Pb2+(aq) + 2Cl−(aq) → PbCl2(s)
(D) Pb(NO3)(s) + 2Cl−(aq) → PbCl2(s) + 2NO3(aq)
Free-Response
Answer the following questions considering the chemical and physical properties of ionic substances.
(a) A large majority of the monatomic ions of the representative elements all have one feature in common. What is that feature? Give a specific example to illustrate it.
(b) Write the molecular equation, the ionic equation, and the net ionic equation for the aqueous reaction of potassium chloride with lead nitrate. Indicate the phase of each of the reactants and products, knowing that PbCl2 is insoluble.
(c) What is the name for Cr(NO3)3 and what is the formula for copper(II) sulfate pentahydrate?
(d) Although it has not been mentioned, by analogy, what is the formula for the iodate ion?
(e) What is potassium permanganate used for? Does it have distinguishing physical properties?




