Practice Exercises - Experimental Chemistry - AP Chemistry Premium 2024
Multiple-Choice
1. This ion gives a bright-orange color in a flame test.
(A) Sodium ion
(B) Silver ion
(C) Bromide ion
(D) Sulfide ion
2. This ion is usually oxidized before it is identified.
(A) Sodium ion
(B) Silver ion
(C) Bromide ion
(D) Sulfide ion
3. Which ion(s) can be identified by a characteristic odor?
(A) Sodium ion
(B) Silver ion and bromide ion
(C) Bromide ion
(D) Sulfide ion
4. Which ion is most commonly identified as a white precipitate that dissolves in ammonia?
(A) Sodium ion
(B) Silver ion
(C) Bromide ion
(D) Sulfide ion
5. A 35.25 mL sample is needed. The best piece of glassware to use is
(A) a buret
(B) a graduated cylinder
(C) a volumetric flask
(D) a volumetric pipet
6. Oxygen formed from the decomposition of KClO3 in a lab experiment is collected by
(A) upward displacement of air
(B) displacement of water
(C) downward displacement of air
(D) displacement of mercury
7. A very fine precipitate is best isolated by
(A) distillation
(B) filtration
(C) vacuum filtration
(D) centrifugation
8. Two students each calibrated the same balance using a standard mass of 0.1000 g. Each repeated the measurement three times, yielding the following data.
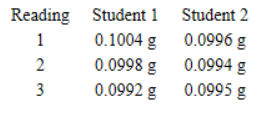
If we compare the two sets of data,
(A) student 2 is more accurate but student 1 is more precise
(B) student 1 is more accurate but student 2 is more precise
(C) student 1 is more precise and more accurate than student 2
(D) student 2 is more precise and more accurate than student 1
9. A 1.00 M solution of NaOH is prepared by weighing exactly 40.0 g of NaOH and adding it to exactly 1000 mL of distilled water at room temperature. Which of the following is most likely to be the largest source of error using the above procedure?
(A) Water should be added to the solute until the desired volume of solution is reached.
(B) The room temperature is not 25 °C.
(C) The glassware is incorrectly calibrated.
(D) NaOH absorbs atmospheric water.
10. The vapor pressure of a liquid is measured at several temperatures. When a graph of the data is made,
(A) temperature is the x-axis since it is the dependent variable
(B) pressure is the y-axis since it is the dependent variable
(C) 1/T is the x-axis because of Raoult’s law
(D) mole fraction is the x-axis and is the independent variable
11. If a student wished to transfer a coarsely powdered solid from a stock bottle to a small test tube, what would be the best method?
(A) Use an evaporating dish.
(B) Transfer it from the stock bottle directly.
(C) Use a thin-stemmed funnel.
(D) Use a creased square of paper.
12. Which measurement is expressed to the appropriate number of significant figures?
(A) 37 seconds from a timer that is graduated in tenths of a second
(B) 0.985 cm from a ruler that is graduated in millimeters
(C) 24.68 mL from a buret that is graduated in tenths of a milliliter
(D) 7.56 °C from a thermometer that is graduated in degrees
13. Which of the following dissolves in both acids and bases?
(A) CaO
(B) CO2
(C) Al(OH)3
(D) AgCl
.png)
14. A 25.0 mL sample of a monoprotic acid was titrated to the end point with 20.0 mL of 0.200 M NaOH, and the molarity of the acid was calculated as 0.160 M. After the titration was complete, it was noticed that the buret was not clean, and droplets of solution were seen on the inside of the buret. What can be deduced about the molarity of the unknown?
(A) The calculation is wrong, and the molarity is really 0.250 M.
(B) The recorded volume of NaOH is low, and the molarity too high.
(C) The recorded volume of NaOH is high, and the molarity is too high.
(D) The recorded volume of NaOH is low, and the molarity is too low.
15. A sample is brought into a laboratory and mixed with an equal volume of a preservative solution. For analysis a 5.00 mL sample is diluted to 100 mL, and the concentration of chloride ions in the diluted solution is found to be 3.0 × 10-3M. What is the chloride concentration of the sample?
(A) 1.2 × 10-1M
(B) 6.0 × 10-2M
(C) 1.5 × 10-4M
(D) 7.5 × 10-5M
16. Qualitative analysis is performed on a colored solution. Addition of HCl results in a white precipitate that does not dissolve completely in hot water. The solution probably contained
(A) Pb2+ and a transition metal ion
(B) Ag+ and no other cations
(C) Ag+ and possibly an alkali metal ion
(D) a transition metal ion and Ag+
17. A solution is acidified, and a noticeable odor is observed. Which of the following is the most likely source of the odor?
(A) CH4
(B) NH3
(C) CO2
(D) H2S
18. A flame test was performed on a small amount of a metal salt, and the color of the flame was crimson red. Which metal ion was present in the solution?
(A) Ca2+
(B) K+
(C) Sr2+
(D) Li+
19. The salt CrCl3 may be prepared in pure form by
(A) reacting an excess of Cr metal with HCl gas
(B) reacting excess NaCl with Cr2O3
(C) reacting 3 moles of HCl with 1 mole of Cr(OH)3
(D) reacting 1 mole of Na2CrO4 with 3 moles of HCl
20. The most common method for determining the molarity of a solution of an acid is
(A) gravimetric analysis (weighing a precipitate)
(B) titration with a standard base
(C) determination of the specific gravity of the acid
(D) determination of the volume of gas evolved when the solution is reacted with Mg metal
21. A student was performing an experiment to determine the molar mass of an unknown solid acid. The acid was dissolved in water and titrated with a standard solution of NaOH. Which of the following errors in procedure would result in the calculated molar mass being too low?
I. Rinsing the buret with distilled water rather than the standard NaOH solution before titrating.
II. Some of the solid acid sticking to the side of the flask and not dissolving completely.
III. Using a NaOH solution that had absorbed carbon dioxide.
(A) I only
(B) III only
(C) I and III only
(D) I and II only
22. After a buret is filled for a titration, the bubble of air in the tip is not dislodged. What will be the effect?
(A) No error will result if the bubble does not come out during the titration.
(B) The volume recorded will be high if the bubble comes out.
(C) The mass of sample will be low if the bubble comes out.
(D) All of the above will be true.
23. If an error is made during an experiment, the appropriate action is to
(A) stop the experiment and start over again
(B) make a note of the error in the notebook and finish the experiment
(C) tear the page(s) out of the notebook and start over again
(D) adjust the results to correct for the error
24. Which of the following is appropriate when constructing a graph of experimental data?
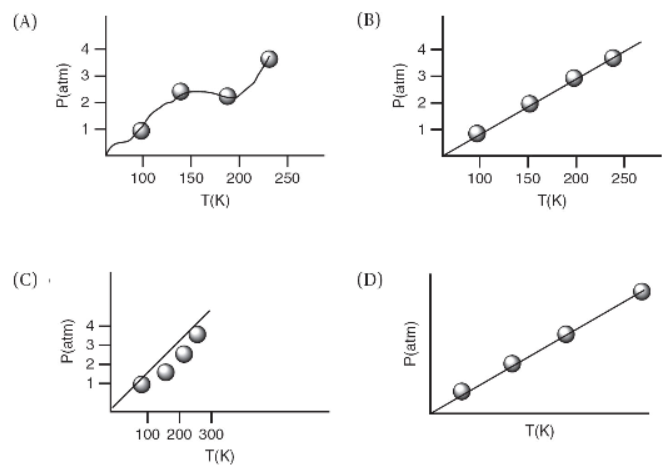
Free-Response
The following questions relate to laboratory work. All of the previous material in this book may be of use in answering these questions.
(a) Explain whether the desired result will be too high, too low, or unaffected in the following situations. Use appropriate equations to support your conclusions.
(i) You pipet 10.0 mL of monoprotic acid and titrate with standard base to calculate the molarity of the acid. You mistakenly blow the last drop of solution from the volumetric pipet each time.
(ii) You determine the molar mass of a gas by determining its density but forget to correct the pressure for the vapor pressure of the water that the gas was collected over.
(iii) In an experiment you dissolve some lead(II) nitrate but do not shake the volumetric flask well. What error results if you pipet from the top of the flask and what will happen if you pipet from the bottom of the flask?
(iv) When you prepare a solid compound, you mistakenly wash the sample with hot water instead of ice water.
(b) Separation methods include decanting, extraction, filtering, distilling, and chromatography. Give an appropriate example of using each method.
(c) Describe how you can detect and eliminate determinate errors.
(d) Flame tests are used to identify some cations, particularly those that do not tend to form complexes or precipitates. List three elements that can be easily determined using a flame test. Explain why flame tests work based on your knowledge of the structure of the atom.




