Practice Exercises - Chemistry of Life - AP Biology Premium 2024
Practice Questions
Multiple-Choice
1. In a water molecule, hydrogen atoms are attached to oxygen atoms through which type of bond?
(A) hydrogen bond
(B) nonpolar covalent bond
(C) polar covalent bond
(D) ionic bond
2. The attraction between the partially positive charge on a hydrogen atom on one water molecule and the partially negative charge on an oxygen atom on another water molecule is called a(n)
(A) hydrogen bond.
(B) nonpolar covalent bond.
(C) polar covalent bond.
(D) ionic bond.
3. Water’s high specific heat is due to
(A) the lower density of solid ice compared to that of liquid water.
(B) the amount of energy required to break the covalent bonds within a water molecule.
(C) the amount of energy required to break the hydrogen bonds between water molecules.
(D) the low electronegativity of oxygen atoms compared to that of hydrogen atoms.
4. Which of the following solutions has the greatest concentration of H+?
(A) stomach acid with a pH of 2
(B) acetic acid with a pH of 3
(C) coffee with a pH of 5
(D) bleach with a pH of 12
5. Solution A has a pH of 4; solution B has a pH of 7. How do the [H+] in these solutions compare?
(A) Solution A has 3 times the [H+] concentration of solution B.
(B) Solution A has 30 times the [H+] concentration of solution B.
(C) Solution A has 1,000 times the [H+] concentration of solution B.
(D) Solution A has 3,000 times the [H+] concentration of solution B.
6. Coastal areas near large bodies of water tend to have more stable climates than inland areas at the same latitude. Which of the following is the property of water that best explains this difference in climate?
(A) high surface tension
(B) high specific heat
(C) capillary action
(D) density of ice
7. Small, lightweight insects can walk on the surface of water, as seen in the following figure:

Which of the following is the property of water that best explains this phenomenon?
(A) high surface tension
(B) high specific heat
(C) capillary action
(D) density of ice
8. Arctic seals and walruses rely on ice floes for survival. Which of the following best explains why these ice floes exist?
(A) high surface tension
(B) high specific heat
(C) capillary action
(D) density of ice
9. Redwood trees, which are over 200 feet tall, can move water upward from their roots to other parts of the tree, despite the downward pull of gravity. Which of the following properties of water best explains this?
(A) high surface tension
(B) high specific heat
(C) capillary action
(D) density of ice
10. In hot weather, humans can cool their body temperature by sweating. Which of the following properties of water makes this possible?
(A) high surface tension
(B) high specific heat
(C) capillary action
(D) density of ice
Short Free-Response
11. On hot summer days, misters will sometimes be used to cool participants at outdoor events.
(a) Describe the property of water that allows misters to have an effective cooling effect.
(b) Explain why the evaporation of water makes the participants in these events more comfortable.
(c) Instead of water, nonpolar oil is spread on the skin. Predict whether this would have a less effective cooling effect, a more effective cooling effect, or the exact same cooling effect as water on the skin.
(d) Using what you know about the comparative properties of water and nonpolar substances, justify your prediction from part (c).
12. Refer to the following figure, which depicts water and methane.
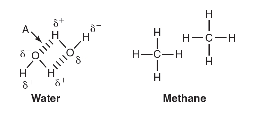
(a) Describe the type of bond indicated by arrow A.
(b) Explain why the bond indicated by arrow A forms between water molecules.
(c) Would an ionic salt dissolve more readily in water or methane? Explain your reasoning.
(d) Plants in arid climates often need to conserve water loss due to evaporation through the leaves of the plant. Some plant species have a waxy, nonpolar cuticle on the outer surface of their leaves. A student claims that this waxy cuticle reduces water loss from the leaves. Support the student’s claim with reasoning.
Long Free-Response
13. Aquatic animals produce carbon dioxide as a product of cellular respiration. Carbon dioxide combines with water to form carbonic acid (H2CO3), which releases hydrogen ions (H+) into solution. Four test tubes (containing 10 mL of water each and different numbers of aquatic snails) are prepared. pH levels were measured in each tube at the beginning of the experiment and after 20 minutes. The results are shown in the following table.
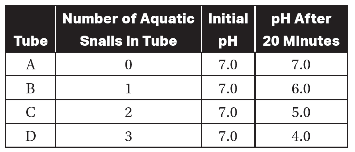
(a) Explain why tubes B, C, and D all had lower pH levels after 20 minutes.
(b) Identify the independent variable and the dependent variable in this experiment.
(c) Analyze the data, and predict which tube (B, C, or D) contained 100 times as many H+ ions as that of tube A after 20 minutes.
(d) Aquatic plants, such as Elodea, perform cellular respiration, but they also perform photosynthesis. Photosynthesis removes carbon dioxide from the water, reducing the amount of carbonic acid. Predict the effect of adding Elodea to all four tubes at the start of the experiment. Justify your prediction.
Practice Questions
Multiple-Choice
1. COVID-19 is a single-stranded RNA virus. Which molecules would most likely be found in a single-stranded RNA virus, such as COVID-19?
(A) adenine, cytosine, deoxyribose, guanine, thymine
(B) adenine, cytosine, deoxyribose, guanine, uracil
(C) adenine, cytosine, ribose, guanine, thymine
(D) adenine, cytosine, ribose, guanine, uracil
2. A scientist conducted an experiment to find out what type of macromolecule a virus injects into a cell. Using radiolabeled atoms, the scientist found that phosphorus from the virus entered the cell but sulfur did not. Which of the following molecules would most likely be injected from this virus into the cell?
(A) carbohydrate
(B) nucleic acid
(C) protein
(D) steroid
3. Which of the following best describes the formation of the primary structure of a protein?
(A) A dehydration reaction forms an ionic bond between the carboxyl group of one amino acid and the amino group of another amino acid.
(B) A dehydration reaction forms a covalent bond between the carboxyl group of one amino acid and the amino group of another amino acid.
(C) A hydrolysis reaction forms an ionic bond between the carboxyl group of one amino acid and the amino group of another amino acid.
(D) A hydrolysis reaction forms a covalent bond between the carboxyl group of one amino acid and the amino group of another amino acid.
4. Which of the following best describes the differences between saturated and unsaturated lipids?
(A) Saturated lipids have at least one C=C double bond and tend to be solid at room temperature. Unsaturated lipids have no double bonds and tend to be liquid at room temperature.
(B) Saturated lipids have at least one C=C double bond, which makes them amphipathic. Unsaturated lipids have no double bonds and are hydrophilic.
(C) Saturated lipids have no C=C double bonds and tend to be solid at room temperature. Unsaturated lipids have at least one C=C double bond and tend to be liquid at room temperature.
(D) Saturated and unsaturated lipids both have C=C double bonds. Saturated lipids are hydrophobic, and unsaturated lipids are hydrophilic.
5. The molecular formula for glucose is C6H12O6. The molecule maltose is formed by a dehydration reaction that links two glucose molecules together. What is the molecular formula for maltose?
(A) C2H4O2
(B) C6H10O5
(C) C12H22O11
(D) C12H24O12
6. In an aqueous environment like the cytosol, the most stable tertiary protein structures would have hydrophilic amino acids in which part of the protein’s structure?
(A) in the interior of the protein, interacting with water in the cytosol
(B) in the interior of the protein, avoiding water in the cytosol
(C) on the surface of the protein, interacting with water in the cytosol
(D) on the surface of the protein, avoiding water in the cytosol
7. Which level of protein structure is formed by peptide bonds between amino acids?
(A) primary
(B) secondary
(C) tertiary
(D) quaternary
8. Which of the following drives the formation of a protein’s secondary structure?
(A) Hydrophobic interactions form between R-groups of amino acids.
(B) Hydrogen bonds form between amino acids in a polypeptide chain.
(C) Disulfide bridges form between amino acids in a polypeptide chain.
(D) Multiple subunits/domains of a protein are connected by covalent bonds.
9. Which of the following is common to both DNA and RNA?
(A) the nitrogenous bases: adenine, cytosine, guanine, and thymine
(B) a double-stranded antiparallel helix
(C) a phosphate group attached to the 5′ carbon
(D) the five-carbon sugar ribose
10. Which of the following correctly describes DNA but not RNA?
(A) It contains adenine, cytosine, guanine, and uracil.
(B) A hydroxyl group is attached to the 5′ carbon.
(C) Nucleotides are linked by phosphodiester bonds.
(D) It contains the five-carbon sugar deoxyribose.
Short Free-Response
11. A student conducts an experiment to compare the melting points of an unsaturated fatty acid and a saturated fatty acid. The data are shown in the table.
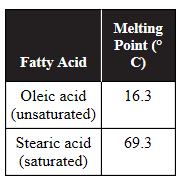
(a) Describe the structural differences between an unsaturated fatty acid and a saturated fatty acid.
(b) Identify the independent variable and the dependent variable in this experiment.
(c) The membrane surrounding cell A contains a much higher percentage of unsaturated fatty acids than the membrane surrounding cell B. Predict which cell would have a more flexible membrane.
(d) Justify your prediction from part (c).
12. The melting temperature (Tm) is defined as the temperature at which 50% of double-stranded DNA is separated into single-stranded DNA. The greater the guanine-cytosine (poly-GC) of the DNA, the higher the Tm compared to DNA with more adenine-thymine (poly-AT) content. The following graph shows the Tm of a poly-AT DNA strand, a poly-GC DNA strand, and DNA from two different organisms (A and B).
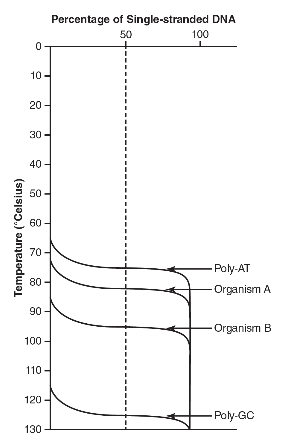
(a) Describe how the Tm of DNA from organism A compares to the Tm of DNA from organism B.
(b) Describe the differences between the Tm of poly-AT and the Tm of poly-GC DNA.
(c) DNA sequencing finds that DNA from organism A has a GC content of 39% and that DNA from organism B has a GC content of 48%. A student claims that DNA from organism C (with a GC content of 55%) would have a Tm greater than 85° Celsius. Using the data from the figure, evaluate the student’s claim.
(d) Explain how these experimental results relate to the structure of DNA.
Long Free-Response
13. Genomes with a higher GC content are more likely to resist denaturation. A plant biologist hypothesized that plants with a higher GC content in their genomes are more likely to be found in colder climates. Four species of plants were examined for the percentage of GC content in their genomes. Brachypodium pinnatum and Dioscorea caucasica are native to areas with cold winters. Juncus inflexus and Carex acutiformis are native to areas with warmer winters. DNA was examined from 50 plants of each species. Results are shown in the table.
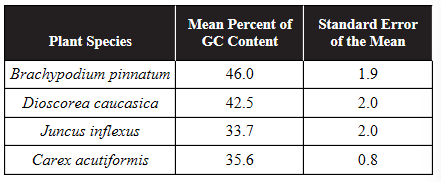
(a) Using what you know about DNA structure, explain why genomes with a higher GC content might be more stable than genomes with a lower GC content.
(b) On the axes provided, construct an appropriately labeled graph of the mean percent of GC content in each species’s genome. Include 95% confidence intervals.
(c) Do the data support the claim that plants in colder climates have a higher GC content in their genomes? Use the data provided to support your answer.
(d) A plant species has a genome with a GC content of 43.4%. Predict whether that plant is more likely to be native to an area with cold winters or an area with warmer winters. Justify your prediction.




