Diagnostic Test 3 - AP Chemistry 2024

1. 40Ca, 39K, and 41Sc all have the same
(A) atomic mass
(B) atomic number
(C) number of neutrons
(D) number of electrons
2. Balance the following skeleton reaction in acid solution using the ion-electron method to obtain a reaction with the smallest possible whole-number coefficients. The sum of all the coefficients in the balanced equation is
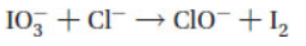
(A) 8
(B) 14
(C) 16
(D) 24
3. The reaction of Br2(g) with Cl2(g) to form BrCl(g) has an equilibrium constant of 15.0 at a certain temperature. If the concentration of BrCl is initially 5.8 × 10−3 moles/liter in the reaction vessel, what will the concentration of BrCl be at equilibrium?
(A) 3.8 × 10−3 mol/L
(B) 5.77 × 10−3 mol/L
(C) 1.97 × 10−3M
(D) 9.9 × 10−4M
4. Which of the following is a balanced chemical equation?
(A) Na2SO4 + Ba(NO3)2 → BaSO4 + NaSO4
(B) AgNO3 + K2CrO4 → Ag2CrO4 + 2KNO3
(C) 5FeCl2 + 8HCl + KMnO4 → 5FeCl3 + MnCl2 + 4H2O + KCl
(D) Al(NO3)3 + 3KOH → Al(OH)3 + KNO3
5. A 50.0 mL sample of 0.0025 M HBr is mixed with 50.0 mL of 0.0023 M KOH. What is the pH of the resulting mixture?
(A) 1.00
(B) 4.00
(C) 5.00
(D) 11.00
6. How many milliliters of 0.250 M KOH does it take to completely neutralize 50.0 mL of 0.150 M H3PO4?
(A) 27 mL
(B) 30.0 mL
(C) 90.0 mL
(D) 270 mL
7. Which of the following pairs have the same electron configuration and are isotopes of each other?
(A) 56Fe2+ and 57Fe3+
(B) 39K+ and 40K+
(C) 24Mg2+ and 25Mg
(D) 40Ca2+ and 40Ar
8. In acid solution the bromate ion, 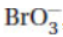 , can react with other substances, resulting in Br2. Balance the half-reaction for bromate ions forming bromine. The balanced half-reaction has
, can react with other substances, resulting in Br2. Balance the half-reaction for bromate ions forming bromine. The balanced half-reaction has
(A) 6 electrons on the left
(B) 6 electrons on the right
(C) 3 electrons on the left
(D) 10 electrons on the left
9. The quantum number ℓ signifies the
(A) relative distance of the electron from the nucleus
(B) orientation in space of a particular orbital
(C) shape of an orbital
(D) spin of the electron
10. When an ideal gas is allowed to expand isothermally, which one of the following is true?
(A) q = 0
(B) w = 0
(C) E = 0
(D) q = −w
11. Which pair of reactants will have no net ionic equation (that is, all the ions cancel)?
(A) Na2SO3 + FeCl2
(B) CaCl2 + MnBr2
(C) NH4I + Pb(NO3)2
(D) KOH + HClO4
12. The rate law for the reaction of 2A + B → 2P is
(A) impossible to determine without experimental data
(B) [A]2[B]
(C) k[A]2[B]
(D) second order with respect to A
13. Hund’s rule requires that
(A) no two electrons can have the same four quantum numbers
(B) no two electrons with the same spin can occupy an orbital
(C) no two electrons can occupy separate orbitals
(D) no two electrons can pair up if there is an empty orbital at the same energy level available
14. Which is a conjugate acid–base pair in the following equation?
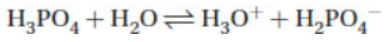
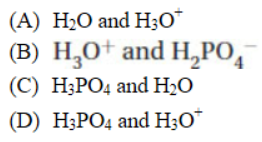
15. The collision theory of reaction rates does not include
(A) the number of collisions per second
(B) the transition state
(C) the energy of each collision
(D) the orientation of each collision
16. A 2.35-gram sample containing chloride ions was dissolved in water, and the chloride ions were precipitated by adding silver nitrate (Ag+ + Cl− → AgCl). If 0.435 g of precipitate was obtained, what is the percentage of chlorine in the sample?
(A) 4.60%
(B) 10.8%
(C) 18%
(D) 43.5%
17. Given the two standard reduction equations and their potentials below, write the thermodynamically favored chemical reaction and its standard cell potential.
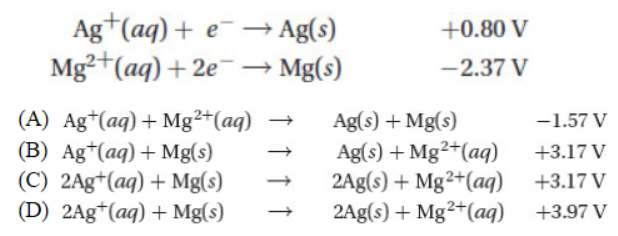
18. Which of the following has an octet of electrons around the central atom?
(A) BF3
(B)
(C) PF5
(D) SF6
19. What is the pH of a solution made by dissolving 0.0300 mol of sodium ethanoate in enough water to make 50 mL of solution (Ka for ethanoic acid is 1.8 × 10−5)?
(A) 4.74
(B) 7.00
(C) 9.26
(D) 11.02
20. The weight percent of sodium hydroxide dissolved in water is 50%. What is the mole fraction of sodium hydroxide?
(A) 31.0%
(B) 0.164
(C) 0.311
(D) 0.500
21. Which of the following representations is most useful for measuring the rate of a chemical reaction?
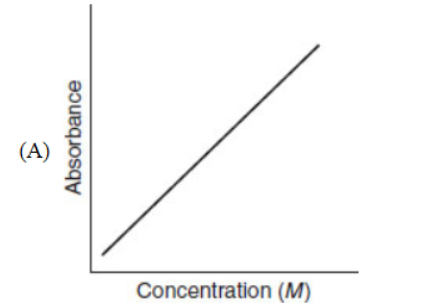
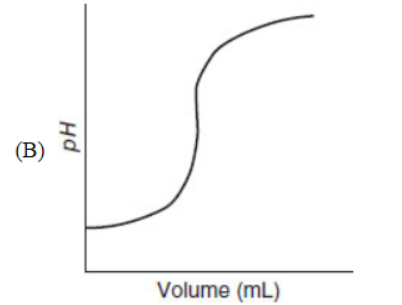
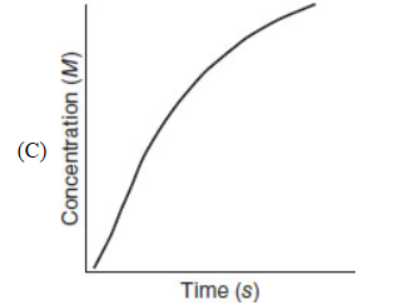
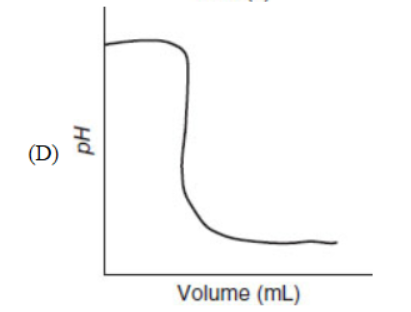
22. Carbon exists in various forms called allotropes. Which of the following is not an allotrope of carbon?
(A) Diamond
(B) Carborundum
(C) Buckminsterfullerene
(D) Graphite
23. What is the molecular mass of a gas that has a density of 2.05 g/L at 26.0 °C and 722 torr?
(A) 4.67 g/mol
(B) 46.7 g/mol
(C) 53.0 g/mol
(D) 2876 g/mol
24. What is the ground state electron configuration for nickel?
(A) 1s22s22p63s23p63d10
(B) 1s22s22p63s24s23d104p4
(C) 1s22s22p63s23p64s24d8
(D) 1s22s22p63s23p64s23d8
25. Under which conditions will a real gas most closely behave as an ideal gas?
(A) High temperature and high pressure
(B) High temperature and low pressure
(C) High volume and high temperature
(D) Low temperature and low pressure
26. The equilibrium constant of a certain reaction is 2.6 × 108 at 25 °C. What is the value of ∆G°?
(A) −48.0 kJ/mol
(B) 20.8 J mol−1
(C) 4.68 × 10−3 kJ/mol
(D) −4.03 kJ mol−1
27. Which of the following geometries corresponds to a substance that has five sigma bonds and one nonbonding pair of electrons on the central atom?
(A) Tetrahedron
(B) Square planar
(C) Octahedron
(D) Square pyramid
28. Carbon dioxide, CO2, when in the form of dry ice is a(n)
(A) network solid
(B) ionic solid
(C) molecular solid
(D) amorphous solid
29. What is the pH of a solution prepared by dissolving 0.1665 mole of hypochlorous acid (HClO) in enough water to make 500 mL of solution? The Ka is 3.0 × 10−4.
(A) 2.00
(B) 1.76
(C) 1.00
(D) 5.4 × 10−3
30. Substances whose Lewis structures must be drawn with an unpaired electron are called
(A) ionic compounds
(B) free radicals
(C) resonance structures
(D) polar molecules
31. Potassium-40 is a minor isotope found in naturally occurring potassium. It is radioactive and can be detected on simple radiation counters. How many protons, neutrons, and electrons does potassium-40 have when it is part of K2SO4?
(A) 21 neutrons, 19 protons, 18 electrons
(B) 20 neutrons, 19 protons, 19 electrons
(C) 21 neutrons, 19 protons, 19 electrons
(D) 19 neutrons, 19 protons, 19 electrons
32. An ideal solution is a
(A) mixture where two solvents cannot be dissolved in all ratios
(B) mixture that has the same physical properties as the individual solvents
(C) mixture where the potential energy of the mixture is the same as that of the individual solvents
(D) mixture that is colorless
33. Which of the following lists the electromagnetic spectral regions in order of decreasing wavelength?
(A) Ultraviolet, visible, infrared, X ray
(B) X ray, visible, ultraviolet, infrared
(C) X ray, ultraviolet, visible, infrared
(D) Infrared, visible, ultraviolet, X ray
34. Sodium fluoride dissolves in water and undergoes hydrolysis. What is the equilibrium expression for the hydrolysis reaction?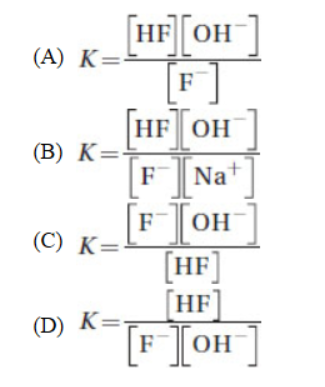
35. Of the following pairs of elements, which pair has the second element with the larger electronegativity based on its position in the periodic table?
(A) Oxygen, chromium
(B) Chlorine, iodine
(C) Calcium, cesium
(D) Boron, nitrogen
36. What is the oxidation number on phosphorus in the compound Na3PO4?
(A) +7
(B) +5
(C) −1
(D) −5
37. Which of the following molecules is a strong electrolyte when dissolved in water?
(A) CH3COOH
(B) HC2H3O2
(C) PCl5
(D) HBr
38. When using the ideal gas law, standard conditions for temperature and pressure are
(A) 0 K and 0 torr
(B) 25 °C and 1 atmosphere pressure
(C) 0 °C and 760 torr
(D) 0 °F and 1 atmosphere pressure
39. The dimerization of NO2(g) to N2O4(g) is an endothermic process. Which of the following will, according to Le Châtelier’s principle, increase the amount of N2O4 in a reaction vessel?
(A) Decreasing the temperature
(B) Increasing the size of the reaction vessel
(C) Adding a selective catalyst
(D) Making the reaction vessel smaller
40. Based on its position in the periodic table, which of the following is expected to have the highest electronegativity?
(A) S
(B) Fe
(C) W
(D) Ag
41. When a solid melts, the entropy and enthalpy changes expected are
(A) positive enthalpy change and positive entropy change
(B) negative entropy change and negative enthalpy change
(C) negative entropy change and positive enthalpy change
(D) negative enthalpy change and positive entropy change
42. What is the mass of one molecule of cholesterol (C27H46O, molecular mass = 386)?
(A) 1.5 × 1021 g
(B) 1.38 × 10−21 g
(C) 6.41 × 10−22 g
(D) 3 × 10−23 g
43. Which of the following pairs of liquids is expected to be immiscible?
(A) H2O and CH3OH
(B) C6H6 and C5H12
(C) C10H22 and CH2CH2CH2OH
(D) CH3CH2NH2 and CH3CH2CH2OH
44. All of the following changes affect the value of the rate constant for a reaction EXCEPT
(A) the addition of a catalyst
(B) decreasing the temperature
(C) decreasing the activation energy
(D) increasing or decreasing the concentration of the reactants
45. Argon can be liquefied at low temperature because of
(A) dipole-dipole attractive forces
(B) hydrogen bonding
(C) instantaneous and induced dipoles
(D) the very low temperature




