Diagnostic Test 2 - AP Chemistry 2024

1. A certain beam of monochromatic light has a frequency of 6.00 × 1014 hertz. The wavelength of this radiation is __________, and this wavelength and frequency reside in the __________ of the electromagnetic spectrum. (The speed of light is 3.00 × 108 m s−1.)
(A) 500. nm visible region
(B) 200. nm ultraviolet region
(C) 2,000,000 m. radio wave region
(D) 500. pm X-ray region
2. Of the following oxo acids, which is predicted to be the strongest acid?
(A) HBrO
(B) HClO
(C) HIO
(D) HClO3
3. What is the equilibrium expression for the reaction below?
C3H8(l) + 5O2(g) → 3CO2(g) + 4H2O(l)
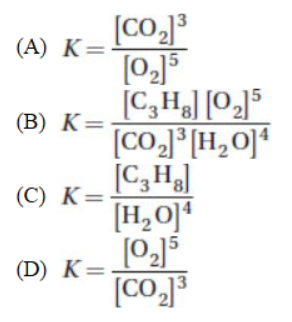
4. The rate law may be written using stoichiometric coefficients for which of the following?
(A) Precipitation reactions
(B) Acid–base reactions
(C) Elementary processes
(D) Solubility reactions
5. A gas in a 5.0 L container under 2.5 atm pressure is allowed to expand to fill a new container in which the pressure has decreased to 1.0 atm. What is the new volume of the gas?
(A) 12.5 L
(B) 10.0 L
(C) 2.0 L
(D) 0.50 L
6. A solution is prepared by dissolving 36.5 grams of Ni(NO3)2 in enough water to make 250 mL of solution. What is the molarity of this solution?
(A) 0.500 molar
(B) 0.800 mol/L
(C) 3.3 molar
(D) 5.00 × 10−3 molar
7. Hard materials such as silicon carbide, used for grinding wheels, are said to be examples of
(A) ionic crystals
(B) network crystals
(C) metallic crystals
(D) molecular crystals
8. The equilibrium constant, Kc, for the dissociation of HI into hydrogen gas and iodine vapor is 21 at a certain temperature. What will be the molar concentration of iodine vapor if 15 grams of HI (the molar mass of HI is 127.90 g mol−1) gas is introduced into a 12.0-L flask and allowed to come to equilibrium?
(A) 4.58 mol/L
(B) 0.00687 mol NaOH L−1
(C) 4.4 × 10−3M NaOH
(D) 9.76 × 10−3M NaOH
9. What is the molarity of a sodium hydroxide solution that requires 42.6 mL of 0.108 M HCl to neutralize 40.0 mL of the base?
(A) 1.64 M NaOH
(B) 0.400 mol/L
(C) 0.115 M
(D) 0.0641 M
10. Changing which of the following will change the numerical value of the equilibrium constant?
(A) The pressure of reactants
(B) The pressure of products
(C) The temperature of the reaction
(D) The total mass of the chemicals present
11. Of the reactions below, which is a decomposition reaction?
(A) C7H8O2(l) + 8O2(g) → 7CO2(g) + 4H2O(l)
(B) 2KClO3(s) → 2KCl(s) + 3O2(g)
(C) 2Cr(s) + 3Cl2(g) → 2CrCl3(s)
(D) 6Li(s) + N2(g) → 2Li3N(s)
12. Helium effuses through a pinhole 5.33 times faster than an unknown gas. That gas is most likely
(A) CO2
(B) CH4
(C) C5H12
(D) C8H18
13. The Pauli exclusion principle states that
(A) no two electrons can have the same energy
(B) no two electrons can have the same four quantum numbers
(C) no two electrons can occupy separate orbitals
(D) no two electrons can pair up if there is an empty orbital available
14. Which of the following can form hydrogen bonds?
(A) CH3OCH2CH3
(B) HCN
(C) CH3OCH2Br
(D) CH3NH2
15. Which experiment led to the notion that the atom contains an extremely small, positively charged nucleus
(A) Millikan’s oil drop experiment
(B) Rutherford’s gold foil experiment
(C) Thomson’s cathode ray experiment
(D) Dalton’s atomic experiment
16. Which of the following is expected to be a polar molecule?
(A) PCl4F
(B) BF3
(C) CO2
(D) Si(CH3)4
17. All of the following ions have the same electron configuration EXCEPT
(A) Rb+
(B) Se2−
(C) As5+
(D) Sr2+
18. Using a periodic table, predict which of the following is the negative end of the bond written last?
(A) O−N
(B) S−As
(C) F−I
(D) P−Cl
19. What is the molar mass of a monoprotic weak acid that requires 26.3 mL of 0.122 M KOH to neutralize 0.682 gram of the acid?
(A) 682 g mol−1
(B) 212 g mol−1
(C) 147 g mol−1
(D) 4.70 g mol−1
20. Which of the following correctly lists the individual intermolecular attractive forces from the strongest to the weakest?
(A) Induced dipole > dipole-dipole > hydrogen bond
(B) Hydrogen bond > dipole-dipole > induced dipole
(C) Induced dipole > hydrogen bond > dipole-dipole
(D) Dipole-dipole > hydrogen bond > induced dipole
21. A mechanism is a sequence of elementary reactions that add up to the overall reaction stoichiometry. A substance that is produced in one elementary reaction and consumed in another is called
(A) a catalyst
(B) an intermediate
(C) a reactant
(D) a complex
22. How many moles of propane (C3H8) are there in 6.2 g of propane?
(A) 1.4 × 10−1 mol
(B) 7.1 mol
(C) 14.1 mol
(D) 71 mol
23. What is the work involved if a gas in a 2.0-liter container at 2.4 atmospheres of pressure is allowed to expand to 6.0 L against a pressure of 0.80 atmospheres?
(A) −9.62 L atm
(B) −4.8 L atm
(C) −3.2 L atm
(D) +14.4 L atm
24. Monatomic ions of the representative elements are often
(A) found in compounds that are insoluble
(B) very electronegative
(C) isoelectronic with a noble gas
(D) found in highly colored compounds
25. A first-order reaction has a half-life of 34 minutes. What is the rate constant for this reaction?
(A) 3.4 × 10−4 s−1
(B) 2.04 × 10−2 s−1
(C) 2.9 × 10−1 min−1
(D) 34 min
26. The electrolysis of molten magnesium bromide is expected to produce
(A) magnesium at the anode and bromine at the cathode
(B) magnesium at the cathode and bromine at the anode
(C) magnesium at the cathode and oxygen at the anode
(D) bromine at the anode and hydrogen at the cathode
27. The oxidation number of chlorine in the perchlorate ion, 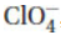 , is
, is
(A) −3
(B) −1
(C) +2
(D) +7
28. A certain reaction has a ∆H° = −43.2 kJ mol−1 and an entropy change ∆S° of +22.0 J mol−1 K−1. What is the value of ∆G° at 800 °C?
(A) +21.2 kJ mol−1
(B) −21.2 kJ mol−1
(C) −66.8 kJ/mol
(D) −2365 kJ mol−1
29. How many electrons, neutrons, and protons are in an atom of 52Cr?
(A) 24 electrons, 24 protons, 24 neutrons
(B) 27 electrons, 27 protons, 24 neutrons
(C) 24 electrons, 28 protons, 24 neutrons
(D) 24 electrons, 24 protons, 28 neutrons
30. The ideal gas law is successful for most gases because
(A) room temperature is high
(B) volumes are small
(C) gas particles do not interact significantly
(D) gases are dimers
31. Which of the following is a conjugate acid-base pair?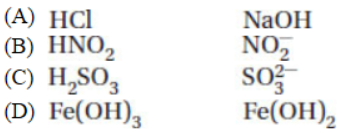
32. The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 signifies the ground state of the element
(A) V
(B) Ti
(C) Co
(D) Ca
33. What is the molar concentration of chloride ions when 35.0 g of MgCl2 (molar mass = 95.21 g/mol) are dissolved in 250. mL of water?
(A) 0.00294 M
(B) 1.47 M
(C) 2.75 M
(D) 2.94 M
34. Which of the following reactions is expected to have the greatest decrease in entropy?
(A) HCl(aq) + NaOH(aq) → NaCl(aq) + H2O
(B) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
(C) C(s) + O2(g) → CO2(g)
(D) 2SO2(g) + O2(g) → 2SO3(g)
35. What is the conjugate acid of the 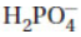 ion?
ion?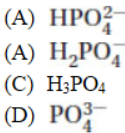
36. A thimble of water contains 4.0 × 1021 molecules. The number of moles of H2O in the thimble is
(A) 2.4 × 1045
(B) 2.4 × 1023
(C) 6.6 × 10−3
(D) 6.6 × 10−23
37. Each resonance form of the nitrate ion, 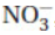 , has how many sigma bonds and how many pi bonds?
, has how many sigma bonds and how many pi bonds?
(A) 1 sigma and 2 pi
(B) 2 sigma and 1 pi
(C) 1 sigma and 1 pi
(D) 3 sigma and 1 pi
38. Twenty-five milligrams of sucrose (C12H22O11) are dissolved in enough water to make 1.00 liter of solution. What is the molarity of the solution?
(A) 7.3 × 10−5
(B) 7.31 × 10−2
(C) 1.36
(D) 73.1
39. Which of the following represents the titration curve of a strong base titrated with a strong acid?
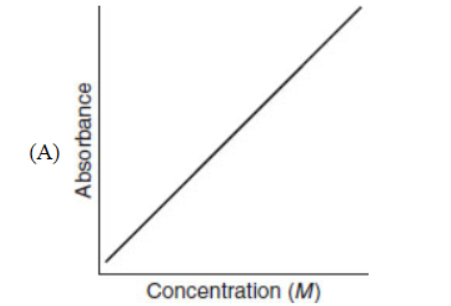
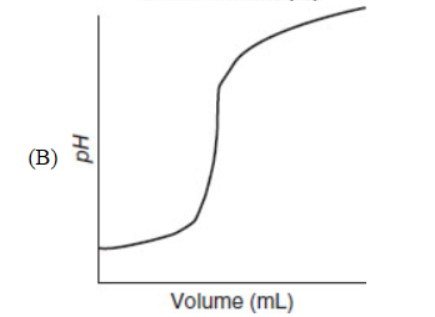
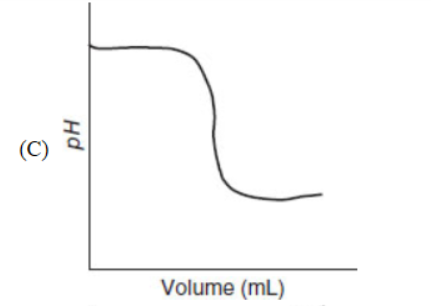
40. A fast reaction rate for a chemical reaction depends on
(A) having a large activation energy
(B) being exothermic
(C) being endothermic
(D) having a small activation energy
41. Which of the following is a reduction half-reaction?
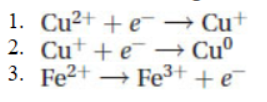
(A) 1 only because copper(II) ions are reduced
(B) 3 only because the iron is reduced
(C) 1 and 2 because they both reduce copper ions
(D) 1 and 3 because they do not have insoluble ions
42. Using a periodic table, determine which of the following pairs is expected to have the largest bond polarity
(A) S−O
(B) P−F
(C) C−B
(D) C−N
43. How many electrons, neutrons, and protons are in an atom of Cr?
(A) 24 electrons, 24 protons, 24 neutrons
(B) 27 electrons, 27 protons, 24 neutrons
(C) 24 electrons, 28 protons, 24 neutrons
(D) More information is needed to answer this question.
44. Which of the following is incorrectly named?
(A) CaCl2 calcium chloride
(B) Fe(NO3)3 iron(III) nitrate
(C) AlBr3 aluminum tribromide
(D) K2Cr2O7 potassium dichromate
45. As the atomic number increases within a period, what happens to the atomic radius of an atom?
(A) It generally increases.
(B) It remains the same.
(C) It generally decreases.
(D) It increases and then decreases.




