Diagnostic Test 1 - AP Chemistry 2024

1. What is the coefficient for H2O when the following reaction is correctly balanced?
__Al(OH)3 + __H2SO4 → __Al2(SO4)3 + __H2O
(A) 1
(B) 2
(C) 3
(D) 6
2. The molar heat of vaporization of water is +43.9 kJ. What is the entropy change for the vaporization of water?
(A) 2.78 J mol-1 K-1
(B) 4.184 J mol-1 K-1
(C) 8.49 J mol-1 K-1
(D) 118 J mol-1 K-1
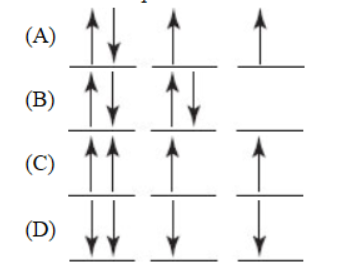
3. Which of the following is a correct representation of the ground state valence p electrons in an atom of sulfur?
4. A liquid element that is a dark-colored, nonconducting substance at room temperature is
(A) mercury
(B) bromine
(C) iodine
(D) bismuth
5. A large positive value for the standard Gibbs free-energy change (∆G°) for a reaction means
(A) the reaction is thermodynamically favored with virtual complete conversion of reactants to products
(B) an extremely fast chemical reaction
(C) a reaction with a very large increase in entropy
(D) none of the above
6. A metal is reacted with HCl to produce hydrogen gas. If 0.0623 gram of metal produces 28.3 mL of hydrogen at STP, the mass of the metal that reacts with one mole of hydrochloric acid is
(A) 493 g
(B) 8.6 g
(C) 9.3 g
(D) 24.7 g
7. An element in its ground state
(A) has all of its electrons in the lowest possible energy levels
(B) is an element as found in nature
(C) is an element that is unreactive and found free in nature
(D) has all of its electrons paired
8. Which following pairs of substances can be used to make a buffer solution?
(A) NaCl and HCl
(B) HC2H3O2 and KC2H3O2
(C) NaBr and KBr
(D) HIO3 and KClO3
9. Which of the following indicates that a reaction is thermodynamically favored?
(A) At equilibrium there are more products than reactants.
(B) The value of ∆G° is greater than zero.
(C) The value of ∆S° is greater than zero.
(D) The value of Keq is less than one.
10. Which of the following is expected to have two or more resonance structures?
(A) CCl2F2
(B) SO3
(C) PF5
(D) H2O
11. Which of the following is a radioactive element?
(A) Na
(B) Cr
(C) Am
(D) Al
12. The units for the rate of a chemical reaction are
(A) L2 mol-2 s-1
(B) mol L−1 s−1
(C) L mol−1 s−1
(D) It depends on the particular reaction.
13. Which of the following is not a good measure of relative intermolecular attractive forces?
(A) Heat of fusion
(B) Boiling point
(C) Vapor pressure
(D) Heat of vaporization
14. Which of the following is expected to be the least soluble in water?
(A) NaBr
(B) NiSO3
(C) CrCl3
(D) Mn(NO3)2
15. The net ionic equation expected when solutions of NH4Br and AgNO3 are mixed together is.png)
16. Less than 1/1000 of the mass of any atom is contributed by
(A) the electrons
(B) the electrons and neutrons
(C) the electrons and protons
(D) the protons and neutrons
17. Which of the following contains the largest number of moles of the indicated metal?
(A) 1.0 g of aluminum
(B) 1.0 g of sodium
(C) 1.0 g of lithium
(D) 1.0 g of silver
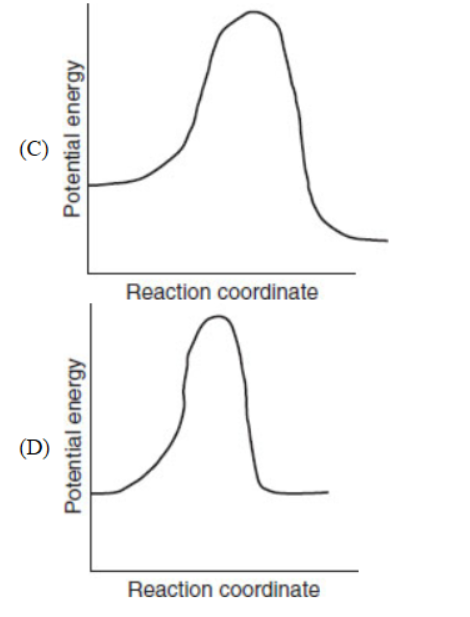
18. Which of the following diagrams represents a potential energy diagram of an endothermic reaction?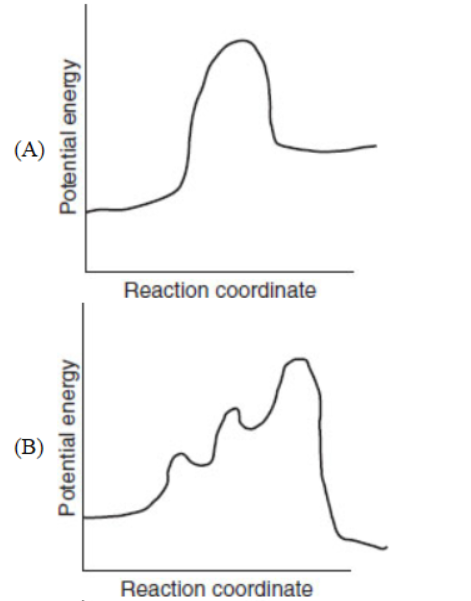
19. The molecule with a tetrahedral shape is
(A) PCl4F
(B) BF3
(C) CO2
(D) CBr4
20. According to the kinetic molecular theory of gases,
(A) the average kinetic energy of a gas particle is directly related to the Kelvin temperature
(B) ideal gas particles do not attract or repel each other
(C) the atoms or molecules of an ideal gas have no volume
(D) (A), (B), and (C) are part of the theory
21. Of the following, the most important experimental information used to deduce the structure of the atom was
(A) the density of each element
(B) the specific heat capacity
(C) the emission spectrum of the elements, particularly hydrogen
(D) the X rays emitted from each element
22. The units for R, the ideal gas law equation constant, may be
(A) L atm mol−1 K−1
(B) J mol−1 K−1
(C) L torr mol−1 K−1
(D) (A), (B), and (C)
23. Which of the following is considered an acid anhydride?
(A) HCl
(B) H2SO3
(C) SO2
(D) Al(NO3)3
24. The standard state for redox reactions includes
(A) the temperature is 25 °C
(B) concentrations of soluble species are 1 molar
(C) partial pressures of gases are 1 atmosphere
(D) all of the above are true
25. What is the theoretical yield of ethyl ethanoate when 100 grams of ethanoic acid is reacted with 100 grams of ethyl alcohol?
CH3COOH + CH3CH2OH → CH3COOCH2CH3 + H2O
(A) 45 g
(B) 147 g
(C) 191 g
(D) 337 g
26. Iron(III) hydroxide has Ksp = 1.6 × 10−39. What is the molar solubility of this compound?
(A) 1.6 × 10−18M
(B) 2.0 × 10−10M
(C) 7.4 × 10−14 mol/L
(D) 9.4 × 10−6 mol/L
27. When 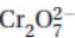 (dichromate ion) is reacted, one of its most common products is Cr3+. What is the oxidation state (oxidation number) of chromium in the dichromate ion? Does reduction or oxidation occur when dichromate ions react to form Cr3+?
(dichromate ion) is reacted, one of its most common products is Cr3+. What is the oxidation state (oxidation number) of chromium in the dichromate ion? Does reduction or oxidation occur when dichromate ions react to form Cr3+?
(A) +3, reduction
(B) +12, reduction
(C) +6, reduction
(D) +6, oxidation
28. Given the electronegativities below, which of the following covalent single bonds is the most polar?
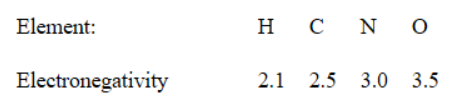
(A) C−H
(B) O−H
(C) N−H
(D) O−C
29. When the following reactants are mixed, what is the correct name and chemical formula for the precipitate that forms?
CuCl2(aq) + Na2CO3(aq) →
(A) Copper(I) carbonate, Cu2CO3
(B) Copper(II) carbonate, Cu2CO3
(C) Copper(I) carbonate, CuCO3
(D) Copper(II) carbonate, CuCO3
30. Which of the following molecules is expected to have the highest normal boiling point?
(A) CH3CH2CH2CH3
(B) CH3CH2CH2CH2OH
(C) CH3CH2CH2CH2Cl
(D) CH3CH2CH2CH2Br
31. Which is correct about the calcium atom?
(A) It contains 20 protons and neutrons.
(B) It contains 20 protons and 20 electrons.
(C) It contains 20 protons, neutrons, and electrons.
(D) All atoms of calcium have a mass of 40.078 u.
32. What is the theoretical yield of iron when 2.00 grams of carbon is reacted with 26.0 grams of Fe2O3?
2Fe2O3 + 3C → 4Fe + 3CO2
(A) 5.8 g
(B) 12.4 g
(C) 30.6 g
(D) 74.6 g
33. Why do vinegar (a dilute solution of ethanoic acid in water) and vegetable oil (long-chain organic acids esterified with glycerol) not mix to form solutions?
(A) The attractive forces in vinegar are much stronger than those in vegetable oil, so the liquids always separate into two phases.
(B) Organic compounds rarely dissolve in water.
(C) Attractive forces in vinegar are mainly hydrogen bonding, while those in vegetable oil are due to instantaneous dipoles.
(D) The unfavorably large endothermic process of “separating” the molecules in the two solutes compared with the energy released when the solutes interact makes a solution thermodynamically unfavored.
34. Which of the following reactions is associated with the normal definition of Kb?
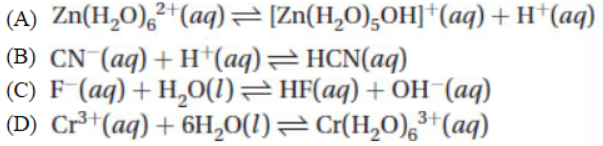
35. Which of the following salts is expected to produce an alkaline solution when one mole is dissolved in one liter of water?
(A) NaClO4
(B) CaCl2
(C) NH4Br
(D) Na2S
36. A 50.0 mL aliquot of a solution containing Al(OH)3 is titrated with 0.0500 molar H2SO4. The end point is reached when 35.0 mL of the sulfuric acid has been added. What is the molarity of the aluminum hydroxide solution?

37. When collecting a gas over water, it is important to
(A) set the temperature at 0 °C
(B) be sure the gas does not burn
(C) wait until the barometer reads 760
(D) correct for the vapor pressure of water
38. How much heat, q, is needed to raise the temperature of 35.5 g of olive oil from 25.0 °C to 75.0 °C? The specific heat of olive oil is 2.0 J/g °C.
(A) 5.33 kJ
(B) 3.55 kJ
(C) 0.888 kJ
(D) 0.282 kJ
39. Sulfur dioxide reacts with oxygen to form sulfur trioxide in the presence of a catalyst. The equilibrium constant, Kp, at a certain temperature is 3.0 × 1022. A 2.0-liter flask has enough SO3 added to it to produce a pressure of 0.789 atm. After the reaction comes to equilibrium, the expected partial pressure of O2 will be
(A) 2.88 × 10−6 torr
(B) 3 × 10−18 mm Hg
(C) 1100 mm Hg
(D) 1.32 × 10−5 torr
40. The melting point of straight-chain hydrocarbons increases as the number of carbon atoms increase. The reason for this is the
(A) increasing mass of the compounds
(B) increasing polarity of the compounds
(C) increasing number of induced dipoles per molecule
(D) increased probability of hydrogen bonds
41. What is the empirical formula of a compound that is 51.9% carbon, 4.86% hydrogen, and 43.2% bromine?
(A) C7H5Br
(B) C6H4Br3
(C) C8H9Br
(D) C12H22Br
42. Which of the following molecules cannot hydrogen bond with molecules identical to itself but can hydrogen bond with one of the molecules above or below it in the following responses?
(A) CH3CH2OH
(B) CH3CH2COOH
(C) CH3CH2CHO
(D) C6H5CHO
43. The standard galvanic cell voltage, E°cell
(A) is equal to E°reduction – E°oxidation
(B) can be used to calculate Keq (if T is known)
(C) can be used to calculate ∆G°
(D) all of the above
44. When will Kp and Kc have the same numerical value?
(A) At absolute zero for all reactions
(B) When the concentrations are at standard state
(C) When the concentrations are all 1.00 molar
(D) When the reaction exhibits no change in pressure at constant volume
45. The rate of a chemical reaction is determined by
(A) the equilibrium constant
(B) the rate-determining or slow step of the mechanism
(C) the reaction vessel pressure
(D) the intermediates formed in the first step




