AP Physics 2 Premium, 2024 - Thermodynamics
1. At constant temperature, an ideal gas is at a pressure of 30 cm of mercury and a volume of 5 L. If the pressure is increased to 65 cm of mercury, the new volume will be
(A) 10.8 L
(B) 2.3 L
(C) 0.43 L
(D) 1.7 L
2. At constant volume, an ideal gas is heated from 75°C to 150°C. The original pressure was 1.5 atm. After heating, the pressure will be
(A) doubled
(B) halved
(C) the same
(D) less than doubled
3. At constant pressure, 6 m3 of an ideal gas at 75°C is cooled until its volume is halved. The new temperature of the gas will be
(A) 174°C
(B) 447°C
(C) −99°C
(D) 37.5°C
4. Water is used in an open-tube barometer. If the density of water is 1,000 kg/m3, what will be the level of the column of water at sea level?
(A) 10.3 m
(B) 12.5 m
(C) 13.6 m
(D) 11.2 m
5. As the temperature of an ideal gas increases, the average kinetic energy of its molecules
(A) increases, then decreases
(B) decreases
(C) remains the sam
(D) increases
6. The product of pressure and volume is expressed in units of
(A )pascals
(B) kilograms per newton
(C) watts
(D) joules
7. Which of the following is equivalent to 1 Pa of gas pressure?
(A) 1 kg/s2
(B) 1 kg · m/s
(C) 1 kg · m2/s2
(D) 1 kg/m · s2
8. What is the efficiency of a heat engine that performs 700 J of useful work from a reservoir of 2,700 J?
(A) 74%
(B) 26%
(C) 35%
(D) 65%
9. The first law of thermodynamics is a restatement of which law?
(A) Conservation of charge
(B) Conservation of energy
(C) Conservation of entropy
(D) Conservation of momentum
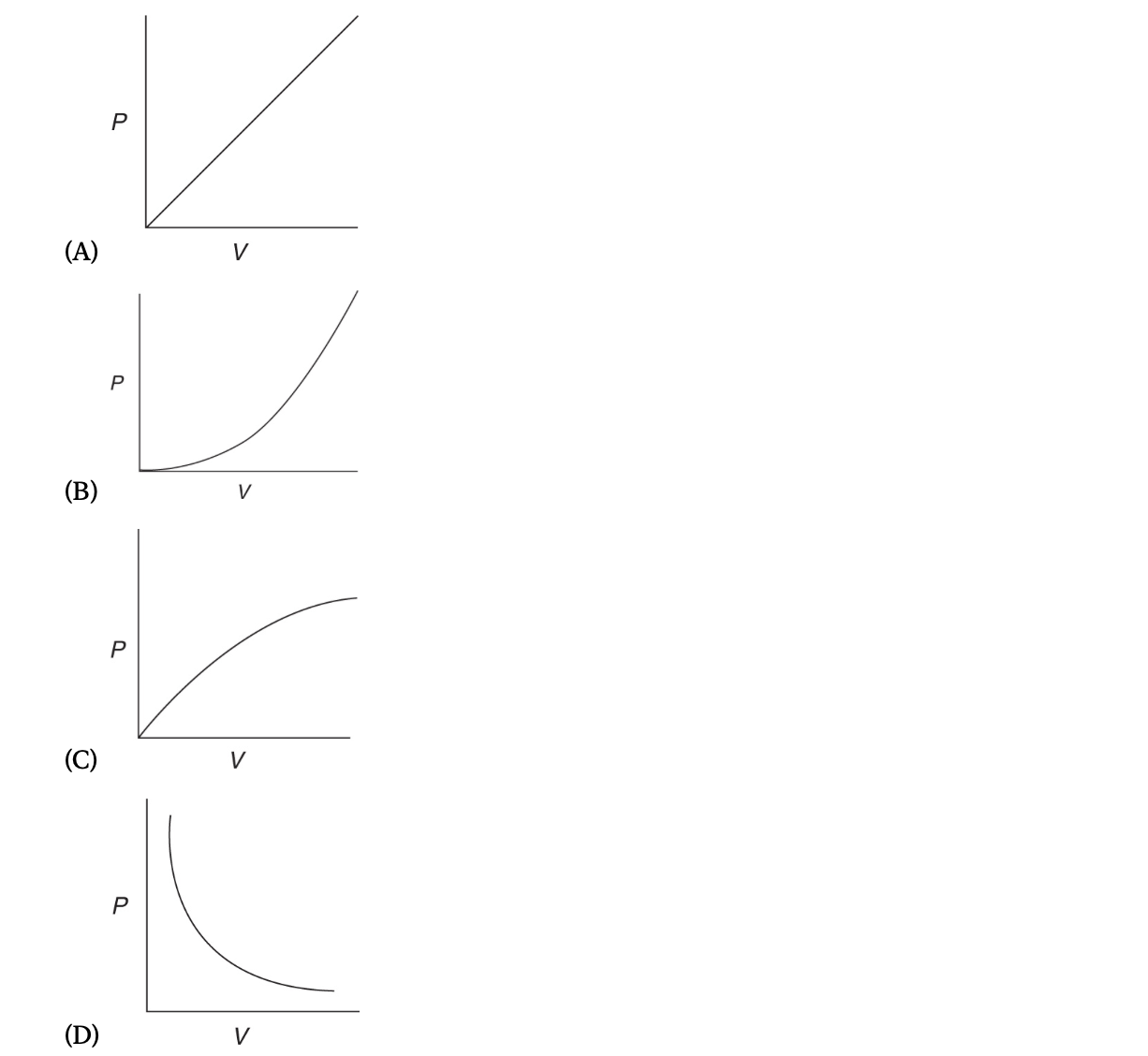
10. Which of the following graphs represents the relationship between pressure and volume for an ideal confined gas at constant temperature?
11.
(a) How many molecules of helium are required to fill a balloon with a diameter of 50 cm at a temperature of 27°C?
(b) What is the average kinetic energy of each molecule of helium?
(c)What is the average velocity of each molecule of helium?
12. An engine absorbs 2,000 J of heat from a hot reservoir and expels 750 J to a cold reservoir during each operating cycle.
(a) What is the efficiency of the engine?
(b) How much work is done during each cycle?
(c) What is the power output of the engine if each cycle lasts for 0.5 s?
13. A monatomic ideal gas undergoes a reversible process as shown in the pressure-versus-volume diagram below:
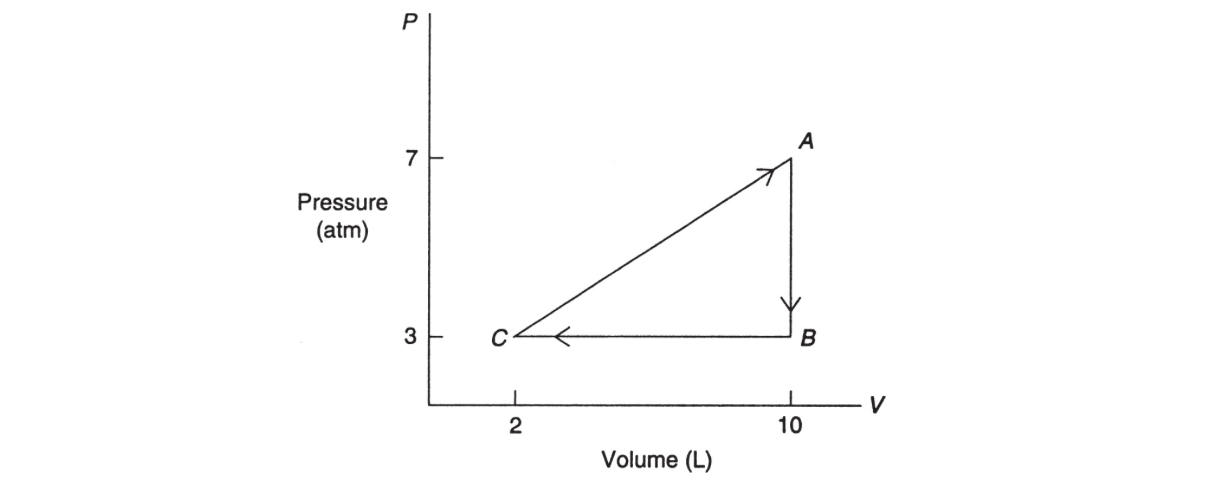
(a) If the temperature of the gas is 500 K at point A, how many moles of gas are present?
(b) For each cycle (AB, BC, and CA), determine the values of ΔW, ΔU, and ΔQ.
14. A glass window has dimensions of 1.2 m high, 1.0 m wide, and 5 mm thick. The outside temperature is 5°C, while the inside air temperature is 15°C.
(a) What is the conductive rate of heat transfer through the window?
(b) How much energy is transferred in one hour?




